 PDF(1763 KB)
PDF(1763 KB)


 PDF(1763 KB)
PDF(1763 KB)
 PDF(1763 KB)
PDF(1763 KB)
非共价法分离光学活性单壁碳纳米管
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Non-Covalent Separation of Optically Active Single-Walled Carbon Nanotubes
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}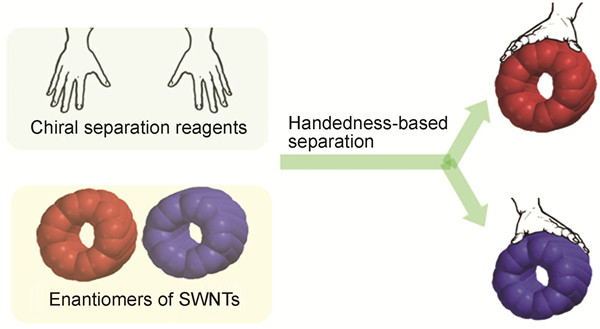
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |