 PDF(4322 KB)
PDF(4322 KB)


 PDF(4322 KB)
PDF(4322 KB)
 PDF(4322 KB)
PDF(4322 KB)
生物质多环碳氢高密度航空燃料合成
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Synthesis of Multi-Cyclic Hydrocarbon High-Density Aviation Fuels from Biomass
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}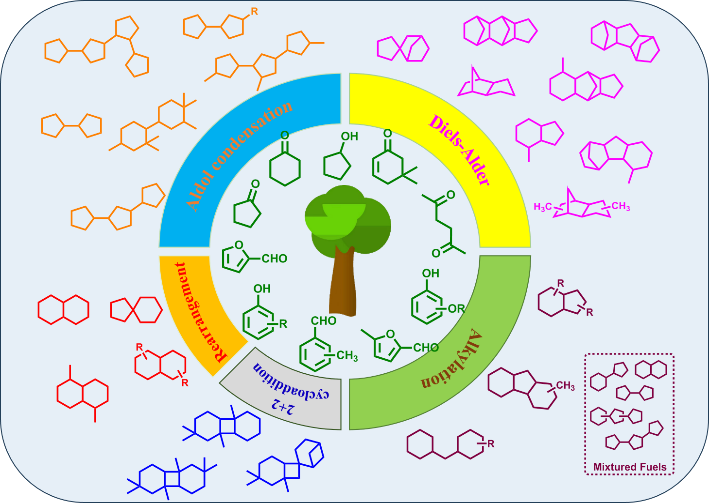
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |