 PDF(11236 KB)
PDF(11236 KB)


杂Diels-Alder 环加成反应级联RAFT聚合
曹如月, 肖晶晶, 王伊轩, 李翔宇, 冯岸超, 张立群
化学进展 ›› 2023, Vol. 35 ›› Issue (5) : 721-734.
 PDF(11236 KB)
PDF(11236 KB)
 PDF(11236 KB)
PDF(11236 KB)
杂Diels-Alder 环加成反应级联RAFT聚合
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Cascade RAFT Polymerization of Hetero Diels-Alder Cycloaddition Reaction
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}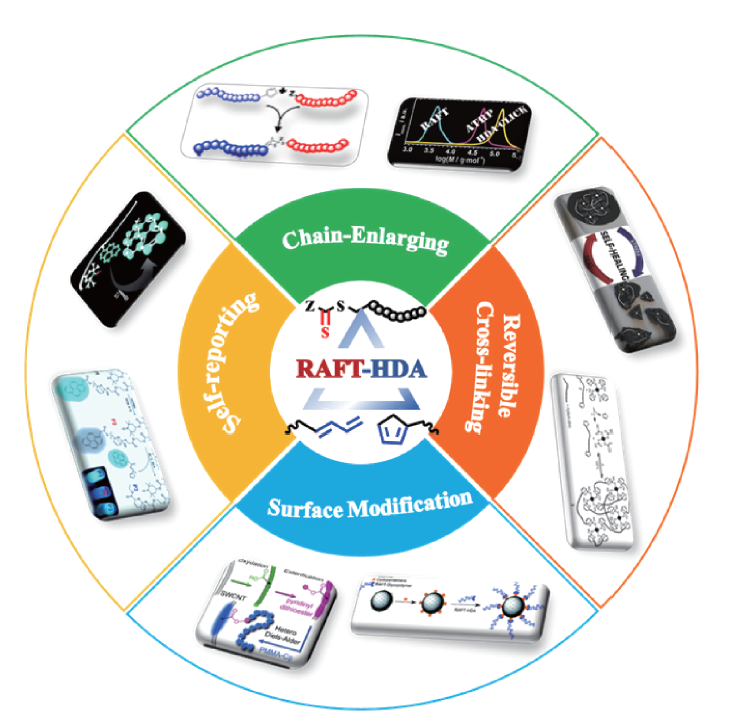
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |