 PDF(8569 KB)
PDF(8569 KB)


多维度非锂无机杂化组分应用于锂电池复合聚合物电解质
马冰怡, 黄盛, 王拴紧, 肖敏, 韩东梅, 孟跃中
化学进展 ›› 2023, Vol. 35 ›› Issue (9) : 1327-1340.
 PDF(8569 KB)
PDF(8569 KB)
 PDF(8569 KB)
PDF(8569 KB)
多维度非锂无机杂化组分应用于锂电池复合聚合物电解质
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Composite Polymer Electrolytes with Multi-Dimensional Non-Lithium Inorganic Hybird Components for Lithium Batteries
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}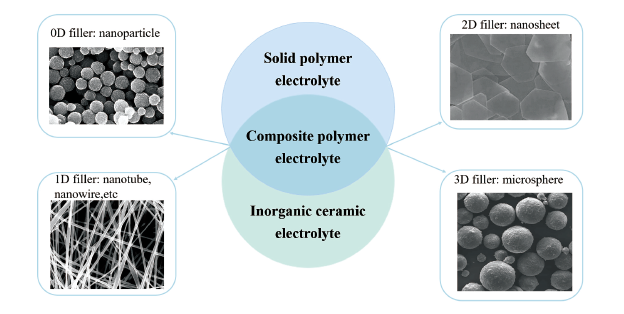
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |