 PDF(19533 KB)
PDF(19533 KB)


 PDF(19533 KB)
PDF(19533 KB)
 PDF(19533 KB)
PDF(19533 KB)
胆甾相液晶螺旋方向的光调控
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Light-Driven Handedness Inversion of Cholesteric Liquid Crystals
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}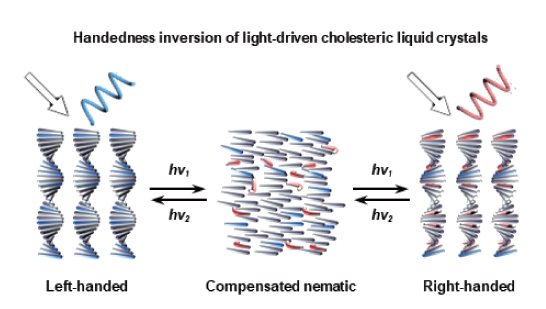
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |