 PDF(11318 KB)
PDF(11318 KB)


基于1,8-萘酰亚胺的多分析物荧光探针的构建和应用
赖燕琴, 谢振达, 付曼琳, 陈暄, 周戚, 胡金锋
化学进展 ›› 2022, Vol. 34 ›› Issue (9) : 2024-2034.
 PDF(11318 KB)
PDF(11318 KB)
 PDF(11318 KB)
PDF(11318 KB)
基于1,8-萘酰亚胺的多分析物荧光探针的构建和应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Construction and Application of 1,8-Naphthalimide-Based Multi-Analyte Fluorescent Probes
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}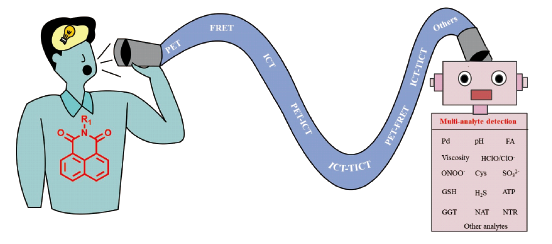
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |