 PDF(6703 KB)
PDF(6703 KB)


 PDF(6703 KB)
PDF(6703 KB)
 PDF(6703 KB)
PDF(6703 KB)
金属氧化物半导体气敏材料抗湿性能提升策略
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Strategies of Improving Anti-Humidity Performance for Metal Oxide Semiconductors Gas-Sensitive Materials
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}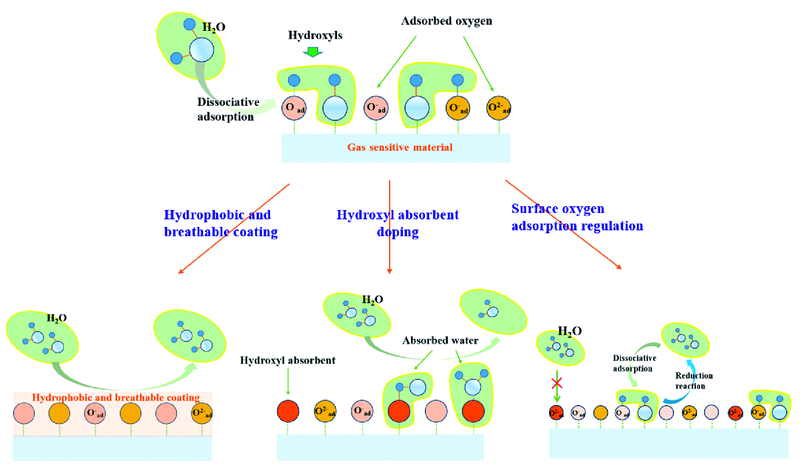
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |