 PDF(2366 KB)
PDF(2366 KB)


 PDF(2366 KB)
PDF(2366 KB)
 PDF(2366 KB)
PDF(2366 KB)
无铅卤系钙钛矿纳米晶:新一代光催化材料
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Lead-Free Halide Perovskite Nanocrystals: A New Generation of Photocatalytic Materials
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}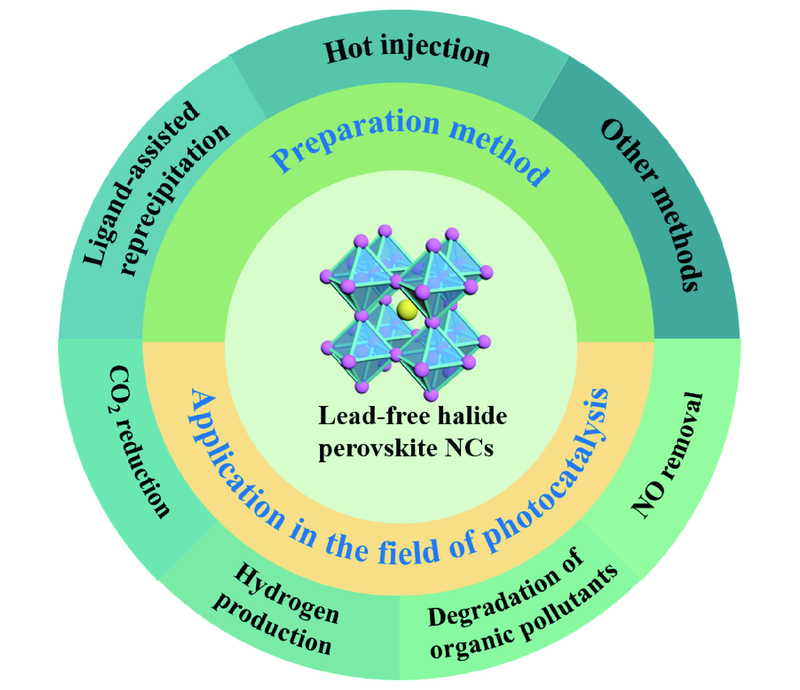
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |