 PDF(1567 KB)
PDF(1567 KB)


质谱光电离/解离技术和生物分子结构鉴定
杨笑宇, 贾珊珊, 张娟, 亓英华, 胡雪雯, 沈宝洁, 钟鸿英
化学进展 ›› 2021, Vol. 33 ›› Issue (12) : 2316-2333.
 PDF(1567 KB)
PDF(1567 KB)
 PDF(1567 KB)
PDF(1567 KB)
质谱光电离/解离技术和生物分子结构鉴定
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Photo Ionization and Dissociation in Mass Spectrometry for Structural Identification of Biological Molecules
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}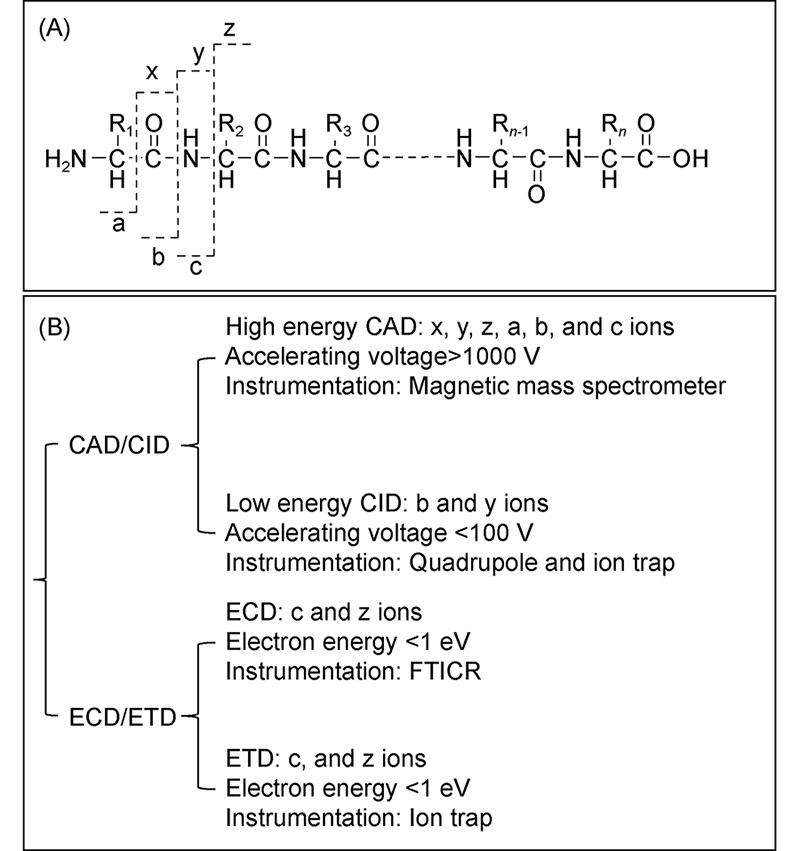
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |