 PDF(11992 KB)
PDF(11992 KB)


基于聚合诱导自组装制备二氧化硅/聚合物纳米复合材料
冯业娜, 刘书河, 张书博, 薛彤, 庄鸿麟, 冯岸超
化学进展 ›› 2021, Vol. 33 ›› Issue (11) : 1953-1963.
 PDF(11992 KB)
PDF(11992 KB)
 PDF(11992 KB)
PDF(11992 KB)
基于聚合诱导自组装制备二氧化硅/聚合物纳米复合材料
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Preparation of SiO2/Polymer Nanocomposites Based on Polymerization-Induced Self-Assembly
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}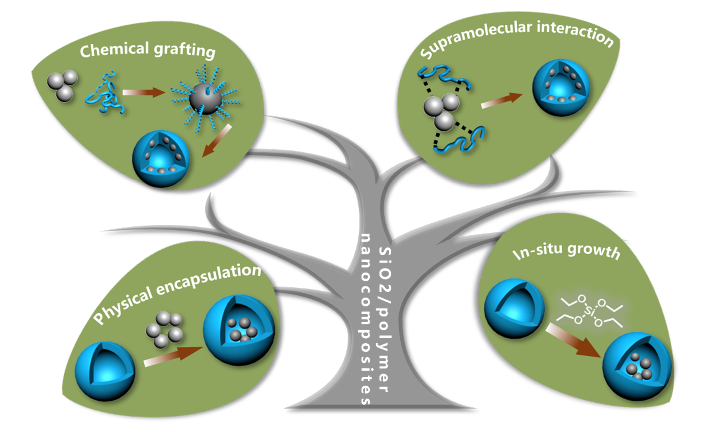
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |