 PDF(13301 KB)
PDF(13301 KB)


 PDF(13301 KB)
PDF(13301 KB)
 PDF(13301 KB)
PDF(13301 KB)
苹果酸——天然产物对映选择性全合成和合成方法学中多用途的手性合成砌块
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Malic acid——A Versatile Chiral Building Block in the Enantioselective Total Synthesis of Natural Products and in Synthetic Methodologies
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}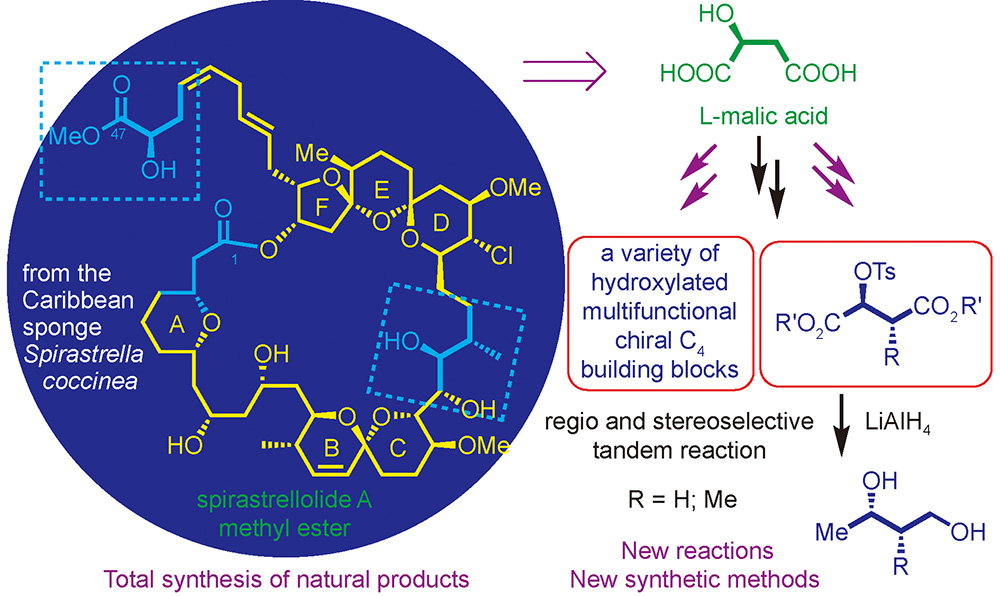
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |