 PDF(10269 KB)
PDF(10269 KB)


 PDF(10269 KB)
PDF(10269 KB)
 PDF(10269 KB)
PDF(10269 KB)
机械研磨条件下凝聚态有机合成探究
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Investigation into Condensed-Matter Organic Synthesis under Mechanical Milling Conditions
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}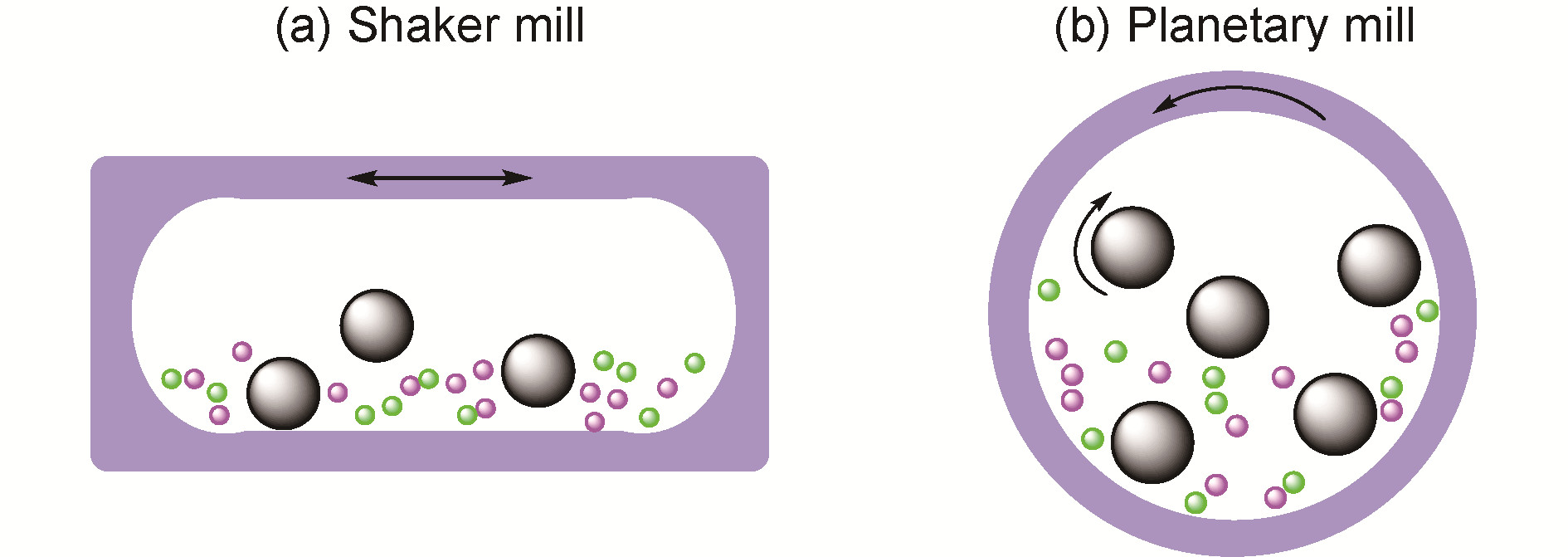
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |