 PDF(9903 KB)
PDF(9903 KB)


 PDF(9903 KB)
PDF(9903 KB)
 PDF(9903 KB)
PDF(9903 KB)
药物输送体系构筑中的超分子组装策略
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Supramolecular Self-Assembly Applied for the Design of Drug Delivery Systems
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}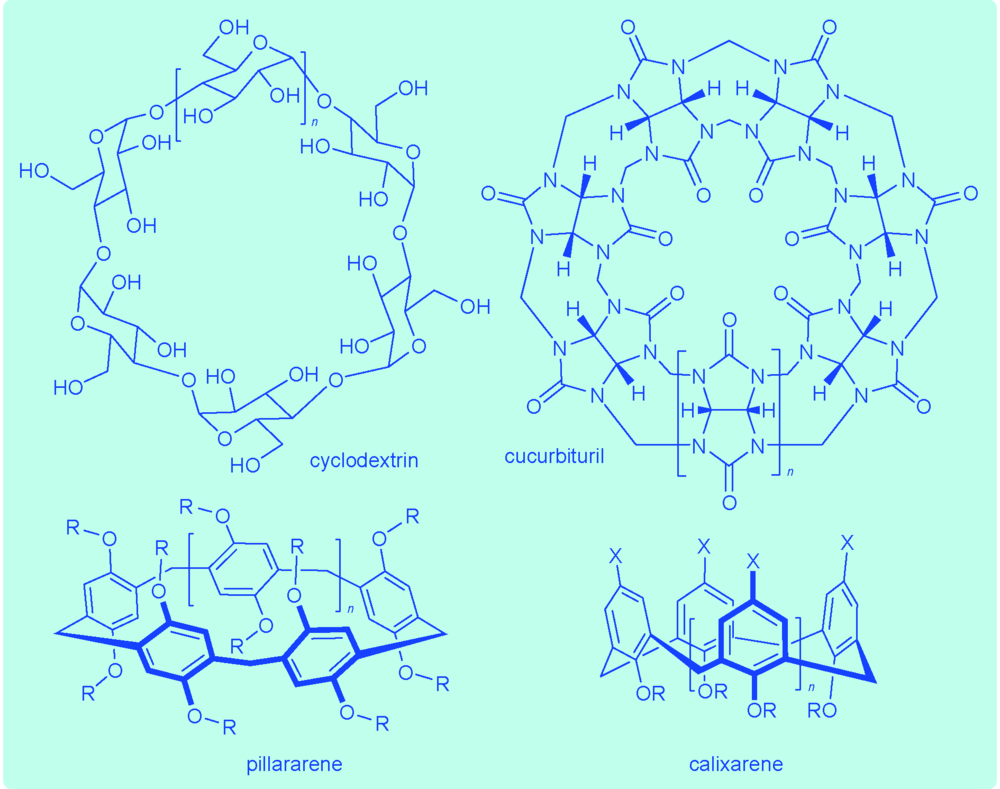
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |