 PDF(12663 KB)
PDF(12663 KB)


 PDF(12663 KB)
PDF(12663 KB)
 PDF(12663 KB)
PDF(12663 KB)
基于铜催化叠氮-炔环加成反应的聚氨酯功能化
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Functionalization of Polyurethane Based on Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}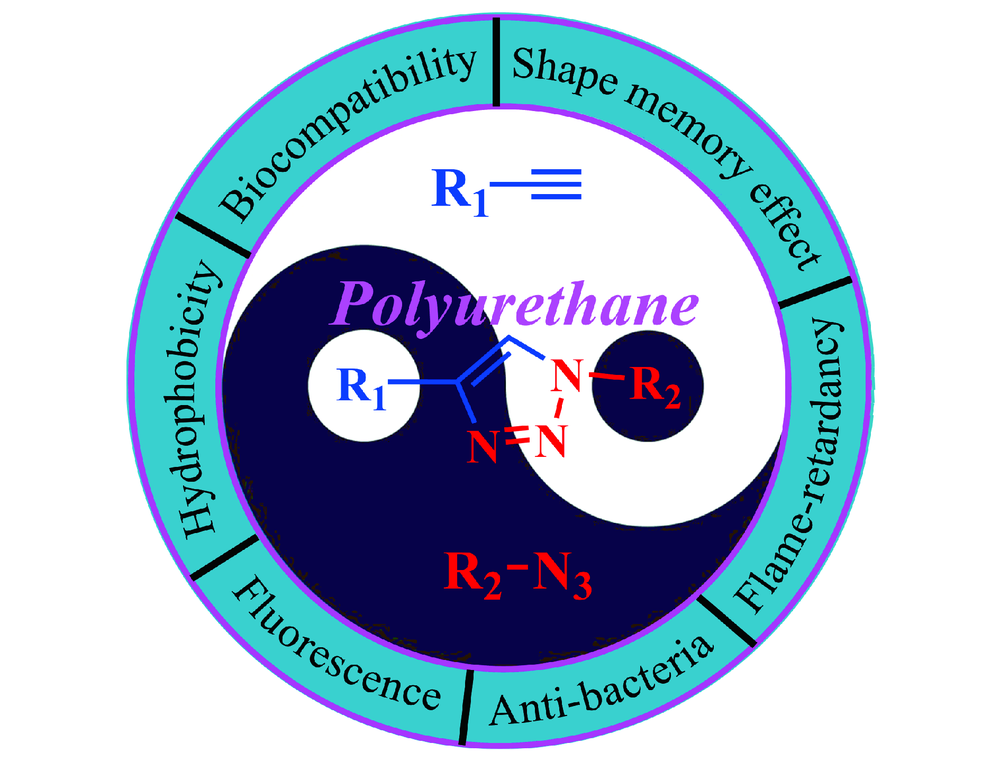
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |