 PDF(7122 KB)
PDF(7122 KB)


 PDF(7122 KB)
PDF(7122 KB)
 PDF(7122 KB)
PDF(7122 KB)
计算模拟研究金属有机骨架材料吸附分离C2、C3烃类气体
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Computational Study on Adsorption and Separation of C2 and C3 Hydrocarbons by Metal-Organic Frameworks
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}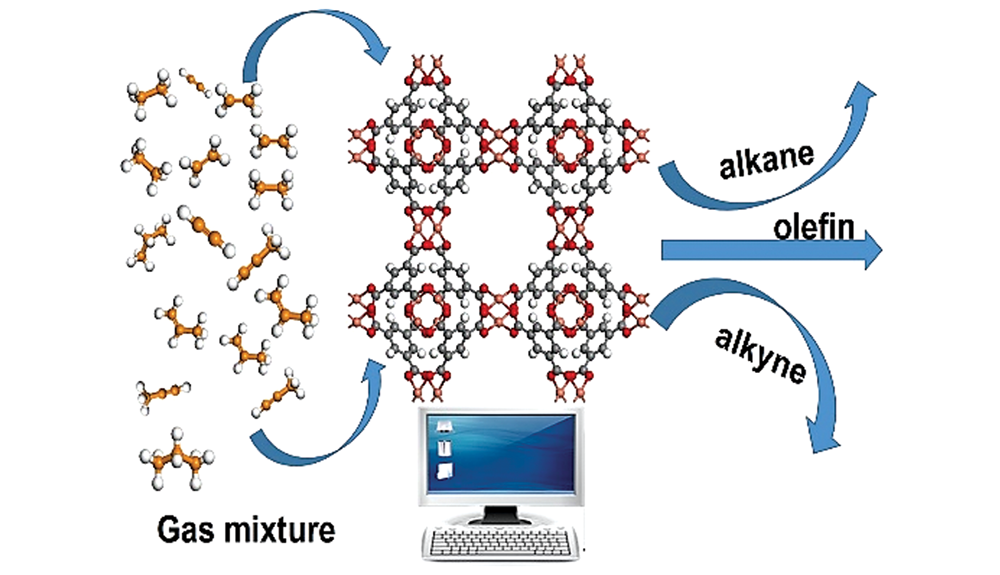
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |