 PDF(11326 KB)
PDF(11326 KB)


 PDF(11326 KB)
PDF(11326 KB)
 PDF(11326 KB)
PDF(11326 KB)
过渡金属硫化物改性锂硫电池正极材料
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Transition-Metal Sulfides Modified Cathode of Li-S Batteries
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}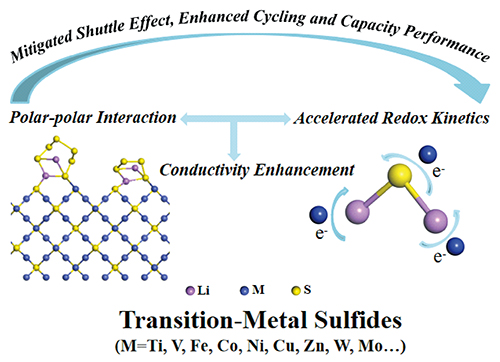
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |