 PDF(7235 KB)
PDF(7235 KB)


可见光催化C(sp 3)-C(sp 3)键的构筑
易享炎, 黄菲, JonathanB.Baell, 黄和, 于杨
化学进展 ›› 2019, Vol. 31 ›› Issue (4) : 505-515.
 PDF(7235 KB)
PDF(7235 KB)
 PDF(7235 KB)
PDF(7235 KB)
可见光催化C(sp 3)-C(sp 3)键的构筑
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}The Formation of C(sp3)-C(sp3) by Visible-Light Photocatalysis
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}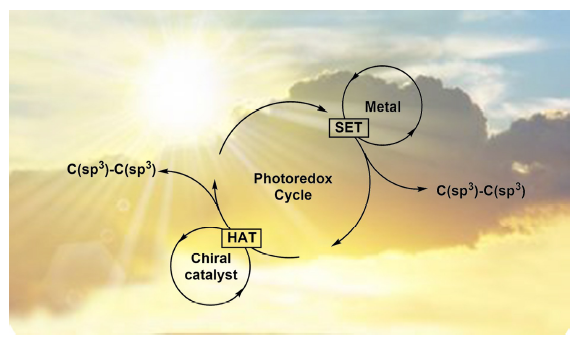
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |