 PDF(5119 KB)
PDF(5119 KB)


 PDF(5119 KB)
PDF(5119 KB)
 PDF(5119 KB)
PDF(5119 KB)
交联型小分子空穴传输材料在溶液工艺制备有机发光二极管中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Application of Solution-Processed Multi-Layer Organic Light-Emitting Diodes Based on Cross-Linkable Small Molecular Hole-Transporting Materials
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}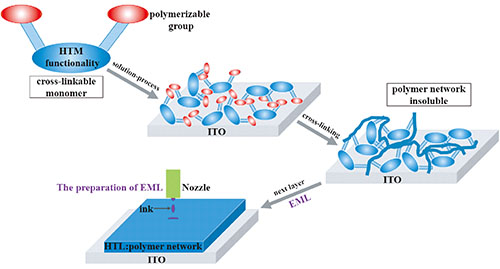
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |