 PDF(6831 KB)
PDF(6831 KB)


氧化石墨烯和石墨烯量子点的两亲性调控及其在Pickering乳液聚合中的应用
卫林峰, 马建中, 张文博, 鲍艳
化学进展 ›› 2017, Vol. 29 ›› Issue (6) : 637-648.
 PDF(6831 KB)
PDF(6831 KB)
 PDF(6831 KB)
PDF(6831 KB)
氧化石墨烯和石墨烯量子点的两亲性调控及其在Pickering乳液聚合中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}The Amphipathy Adjustment of Graphene Oxide and Graphene Quantum Dots and Their Application in Pickering Emulsion Polymerization
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}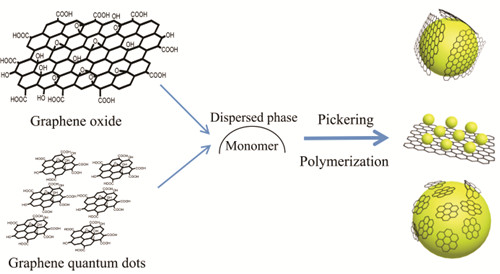
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |