 PDF(9520 KB)
PDF(9520 KB)


 PDF(9520 KB)
PDF(9520 KB)
 PDF(9520 KB)
PDF(9520 KB)
聚酰胺类多肽二级结构模拟物的结构设计与性质分析
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}The Structural Designs and Property Analysis of Polyamide Based Structures as Peptide Secondary Structure Mimics
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}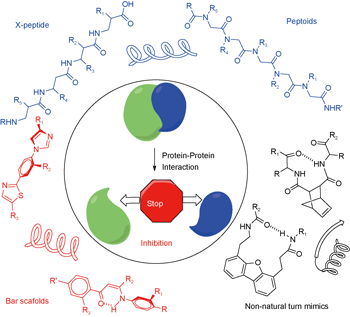
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |