 PDF(28090 KB)
PDF(28090 KB)


 PDF(28090 KB)
PDF(28090 KB)
 PDF(28090 KB)
PDF(28090 KB)
“精准医疗”背景下的分子靶向药物研究——精准药物设计策略浅析
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Contemporary Molecular Targeted Drug in the Context of “Precision Medicine”: An Attempting Discussion of “Precision Drug Design”
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}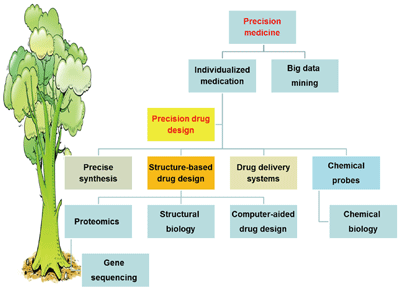
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |