 PDF(24266 KB)
PDF(24266 KB)


 PDF(24266 KB)
PDF(24266 KB)
 PDF(24266 KB)
PDF(24266 KB)
新型烯桥二芳基乙烯光致变色体系
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Photochromic Diarylethenes Based on Novel Ethene Bridges
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}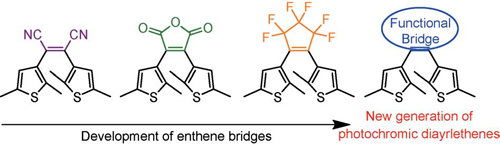
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |