 PDF(1692 KB)
PDF(1692 KB)


 PDF(1692 KB)
PDF(1692 KB)
 PDF(1692 KB)
PDF(1692 KB)
含邻手性碳原子双键亲电加成反应的立体选择性模型
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Stereoselective Models for the Electrophilic Addition on the Double Bond Adjacent to A Chiral Centre
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}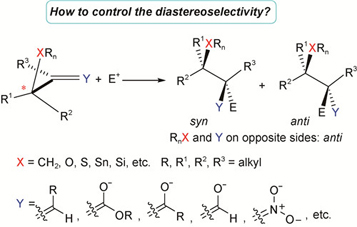
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |