 PDF(1421 KB)
PDF(1421 KB)


聚乙二醇功能化离子液体的制备及其在有机反应中的应用
徐艺凇, 张凤香, 厉嘉云, 白赢, 肖文军, 彭家建
化学进展 ›› 2015, Vol. 27 ›› Issue (10) : 1400-1412.
 PDF(1421 KB)
PDF(1421 KB)
 PDF(1421 KB)
PDF(1421 KB)
聚乙二醇功能化离子液体的制备及其在有机反应中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Preparation and Applications in Organic Reactions of Polyethylene Glycol Functionalized Ionic Liquids
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}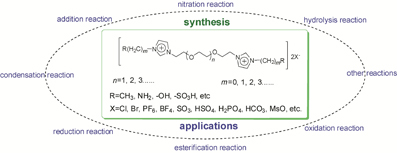
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |