 PDF(8276 KB)
PDF(8276 KB)


苝类化合物研究与应用
王辉, Ponmani Jeyakkumar, Sangaraiah Nagarajan, 孟江平, 周成合
化学进展 ›› 2015, Vol. 27 ›› Issue (6) : 704-743.
 PDF(8276 KB)
PDF(8276 KB)
 PDF(8276 KB)
PDF(8276 KB)
苝类化合物研究与应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Current Researches and Applications of Perylene Compounds
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}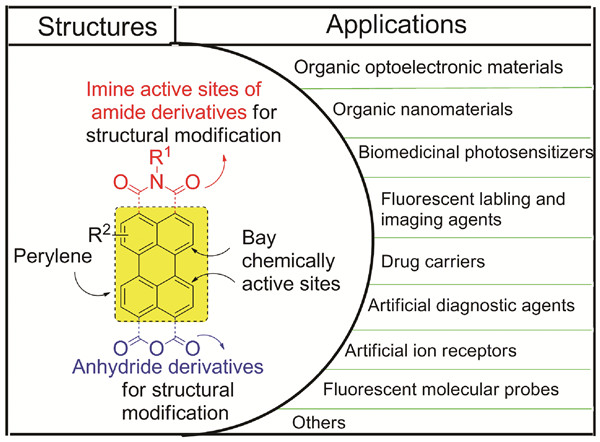
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |