 PDF(1140 KB)
PDF(1140 KB)


 PDF(1140 KB)
PDF(1140 KB)
 PDF(1140 KB)
PDF(1140 KB)
铂配合物类催化剂在γ-氯丙基三氯硅烷合成中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Platinum Complexes Catalyzed Hydrosilylation of Trichlorosilane and Allyl Chloride
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}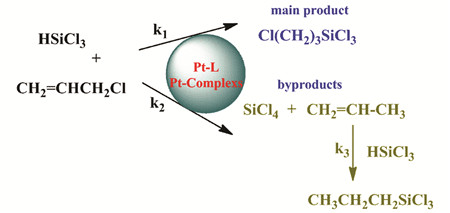
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |