 PDF(16568 KB)
PDF(16568 KB)


 PDF(16568 KB)
PDF(16568 KB)
 PDF(16568 KB)
PDF(16568 KB)
模板导向的亚胺型夹套法在构筑N-杂冠醚类机械互锁分子中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}The Application of Templated-Directed Clipping Approach in Constructing Mechanically Interlocked Molecules Based on N-Hetero Crown Ethers
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}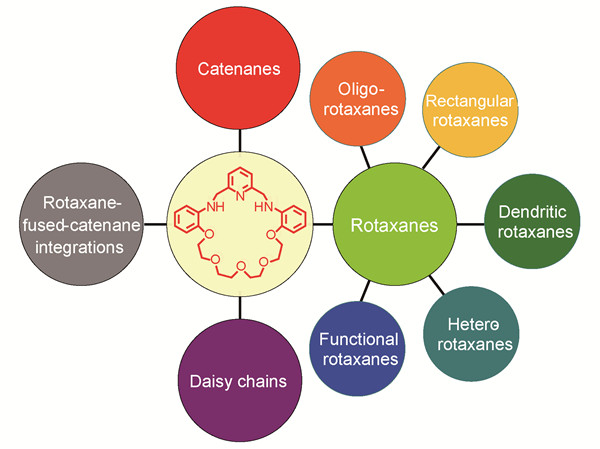
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |