 PDF(5165 KB)
PDF(5165 KB)


 PDF(5165 KB)
PDF(5165 KB)
 PDF(5165 KB)
PDF(5165 KB)
铃木偶联反应构筑卟啉阵列的研究进展
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Recent Development of Constructing Porphyrin Arrays via Suzuki-Miyaura Cross-Coupling Reaction
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}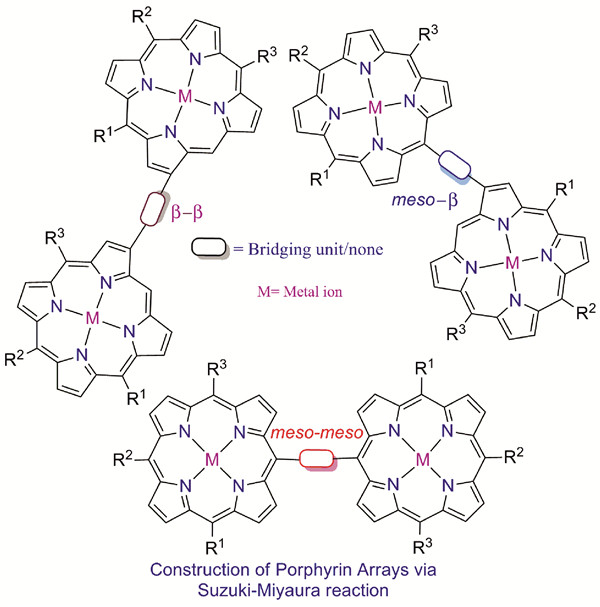
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |