 PDF(7299 KB)
PDF(7299 KB)


 PDF(7299 KB)
PDF(7299 KB)
 PDF(7299 KB)
PDF(7299 KB)
原子尺度研究固体氧化物池氧电极中氧迁移规律
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Atomic-Scale Insights into the Oxygen Ionic Transport Mechanisms of Oxygen Electrode in Solid Oxide Cells:A Review
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}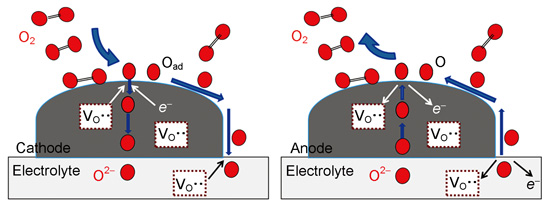
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |