 PDF(1420 KB)
PDF(1420 KB)


 PDF(1420 KB)
PDF(1420 KB)
 PDF(1420 KB)
PDF(1420 KB)
染料敏化太阳能电池掺杂TiO2纳晶光阳极
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Doped Titania Nanocrystalline Photoanodes for Efficiency Improvement of Dye-Sensitized Solar Cells
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}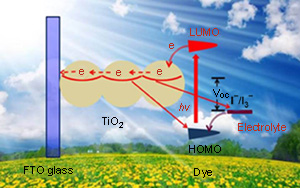
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |