 PDF(5303 KB)
PDF(5303 KB)


 PDF(5303 KB)
PDF(5303 KB)
 PDF(5303 KB)
PDF(5303 KB)
超分子凝胶的手性功能应用:手性分子识别与不对称催化
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Function and Application of Supramolecular Gels:Chiral Molecular Recognition and Asymmetric Catalysis
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}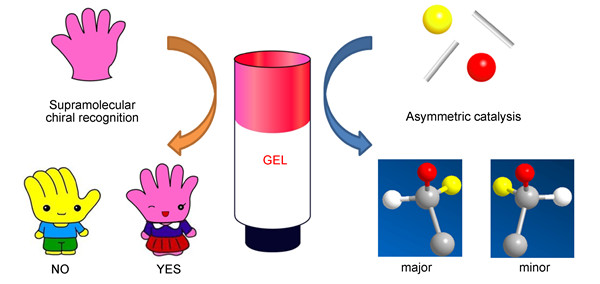
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |