 PDF(1642 KB)
PDF(1642 KB)


 PDF(1642 KB)
PDF(1642 KB)
 PDF(1642 KB)
PDF(1642 KB)
高强度双网络水凝胶的增韧机理
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Toughening Mechanisms of High Strength Double Network Hydrogels
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}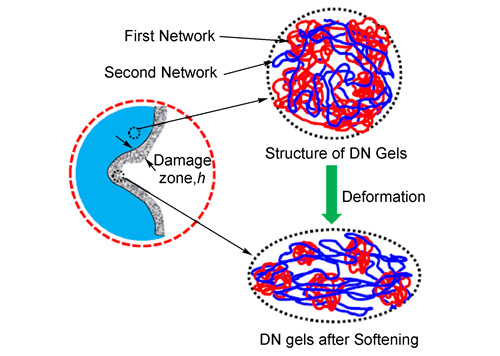
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |