 PDF(12039 KB)
PDF(12039 KB)


 PDF(12039 KB)
PDF(12039 KB)
 PDF(12039 KB)
PDF(12039 KB)
三重和四重氢键体系:设计、结构和应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Triply and Quadruply Hydrogen Bonded Systems:Design, Structure and Application
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}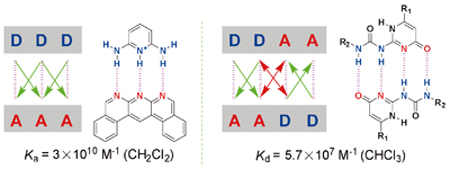
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |