 PDF(4180 KB)
PDF(4180 KB)


 PDF(4180 KB)
PDF(4180 KB)
 PDF(4180 KB)
PDF(4180 KB)
稀土金属配合物催化芳香型乙烯基极性单体立构选择性聚合
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Rare-Earth Metal Complexes-Mediated Stereoselective Polymerization of Aromatic Polar Vinyl Monomers
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}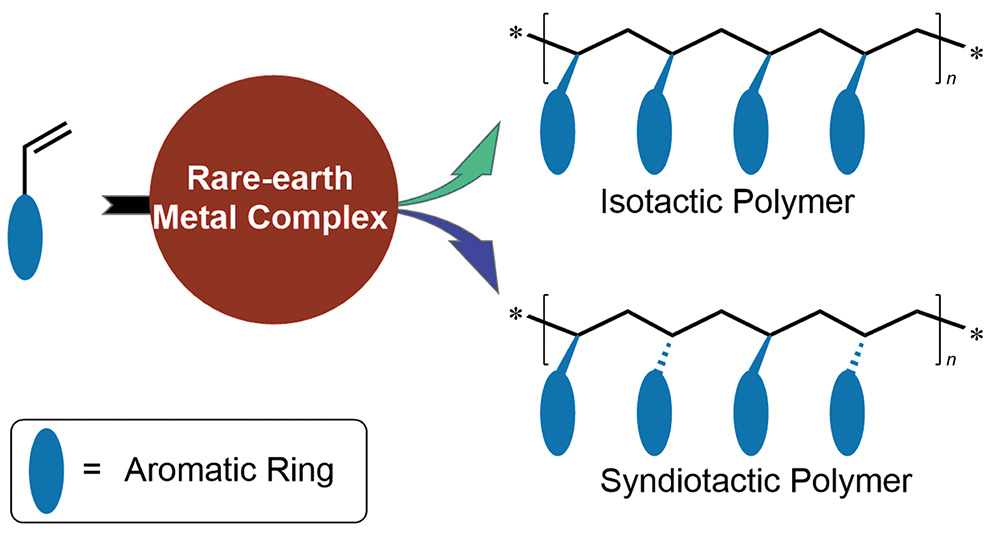
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |