 PDF(14897 KB)
PDF(14897 KB)


 PDF(14897 KB)
PDF(14897 KB)
 PDF(14897 KB)
PDF(14897 KB)
从含有八元环的稠环芳烃到具有负曲率的碳纳米结构:进展与展望
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}From Polycyclic Arenes Containing Eight-Membered Rings to Negatively Curved Nanocarbons: Progress and Outlook
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}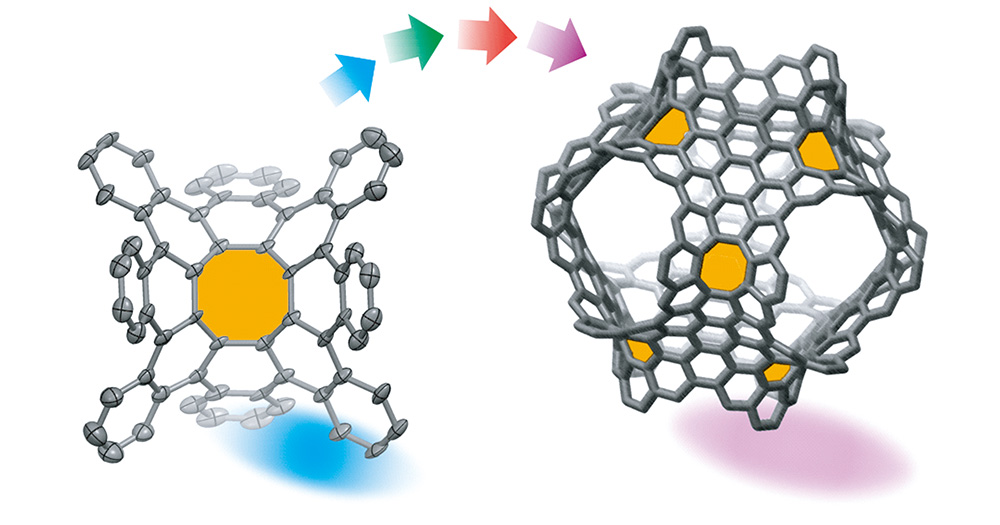
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |