 PDF(19887 KB)
PDF(19887 KB)


 PDF(19887 KB)
PDF(19887 KB)
 PDF(19887 KB)
PDF(19887 KB)
基于全散射技术局域结构确定与凝聚态物质
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Local Structure Determination Based on Total Scattering and Condensed Matter
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}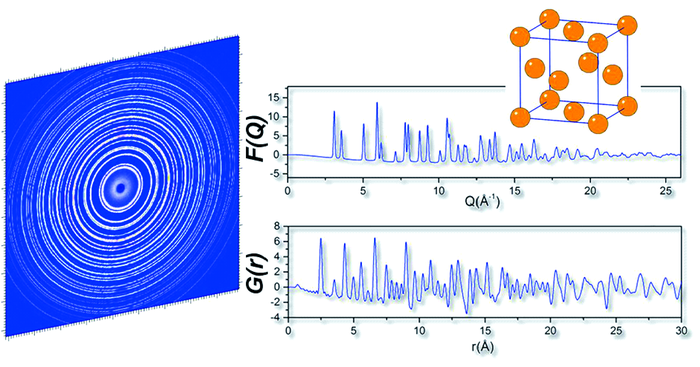
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |