 PDF(9913 KB)
PDF(9913 KB)


 PDF(9913 KB)
PDF(9913 KB)
 PDF(9913 KB)
PDF(9913 KB)
提高非均相芬顿催化活性策略、研究进展及启示
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Strategies, Research Progress and Enlightenment of Enhancing the Heterogeneous Fenton Catalytic Reactivity: A Critical Review
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}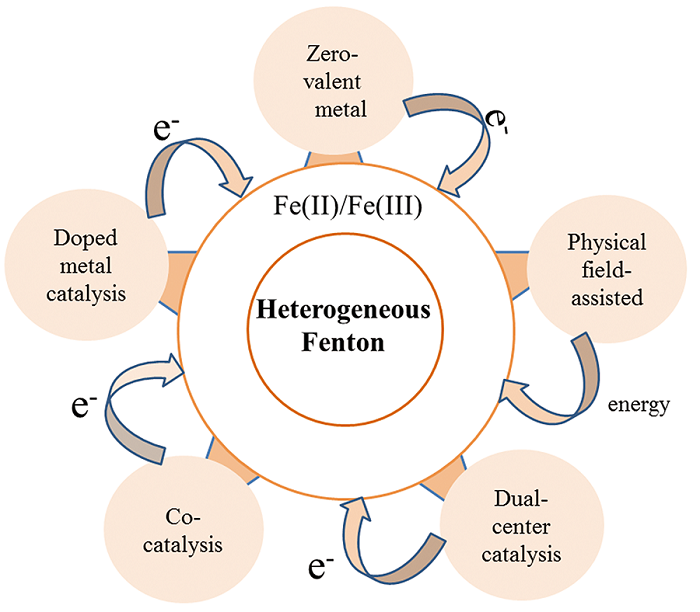
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |