 PDF(13119 KB)
PDF(13119 KB)


 PDF(13119 KB)
PDF(13119 KB)
 PDF(13119 KB)
PDF(13119 KB)
冶金法生产太阳能级硅的除硼方法、技术及工艺
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Boron Removal Method, Technology and Process for Producing Solar Grade Silicon by Metallurgical Method
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}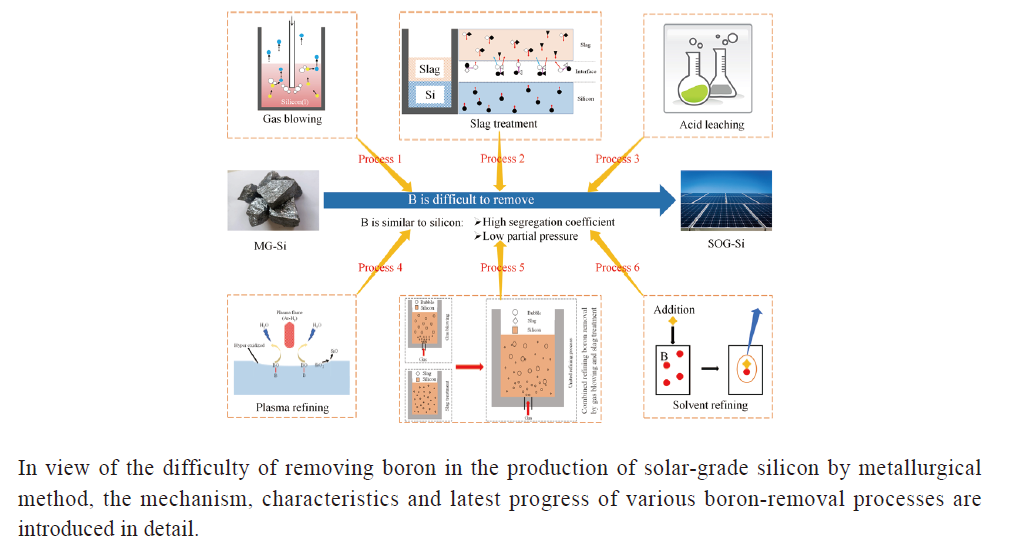
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |