 PDF(9988 KB)
PDF(9988 KB)


以双氧水或氧气为氧化剂的苯羟基化制苯酚的催化反应机理
张明珏, 凡长坡, 王龙, 吴雪静, 周瑜, 王军
化学进展 ›› 2022, Vol. 34 ›› Issue (5) : 1026-1041.
 PDF(9988 KB)
PDF(9988 KB)
 PDF(9988 KB)
PDF(9988 KB)
以双氧水或氧气为氧化剂的苯羟基化制苯酚的催化反应机理
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Catalytic Reaction Mechanism for Hydroxylation of Benzene to Phenol with H2O2/O2 as Oxidants
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}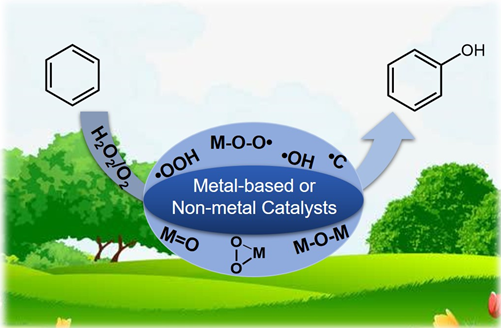
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |