 PDF(7910 KB)
PDF(7910 KB)


 PDF(7910 KB)
PDF(7910 KB)
 PDF(7910 KB)
PDF(7910 KB)
纳米零价铁去除水体中砷的效能与机理
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Performance and Mechanism of Aqueous Arsenic Removal with Nanoscale Zero-Valent Iron
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}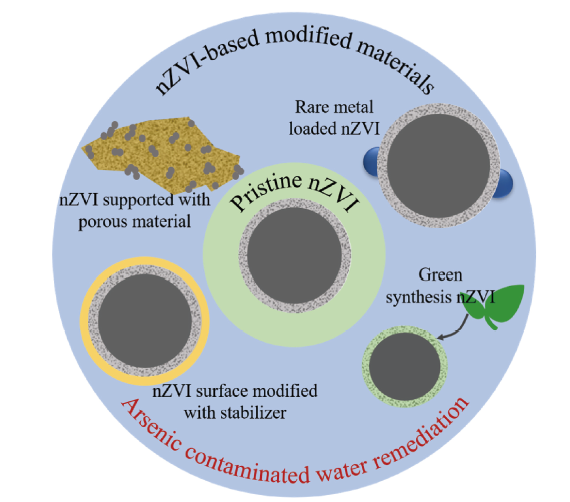
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |