 PDF(12819 KB)
PDF(12819 KB)


金属有机骨架材料在氨低温催化还原氮氧化物反应中的应用
王晓晗, 刘彩霞, 宋春风, 马德刚, 李振国, 刘庆岭
化学进展 ›› 2020, Vol. 32 ›› Issue (12) : 1917-1929.
 PDF(12819 KB)
PDF(12819 KB)
 PDF(12819 KB)
PDF(12819 KB)
金属有机骨架材料在氨低温催化还原氮氧化物反应中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Application of Metal-Organic Frameworks for Low-Temperature Selective Catalytic Reduction of NO with NH3
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}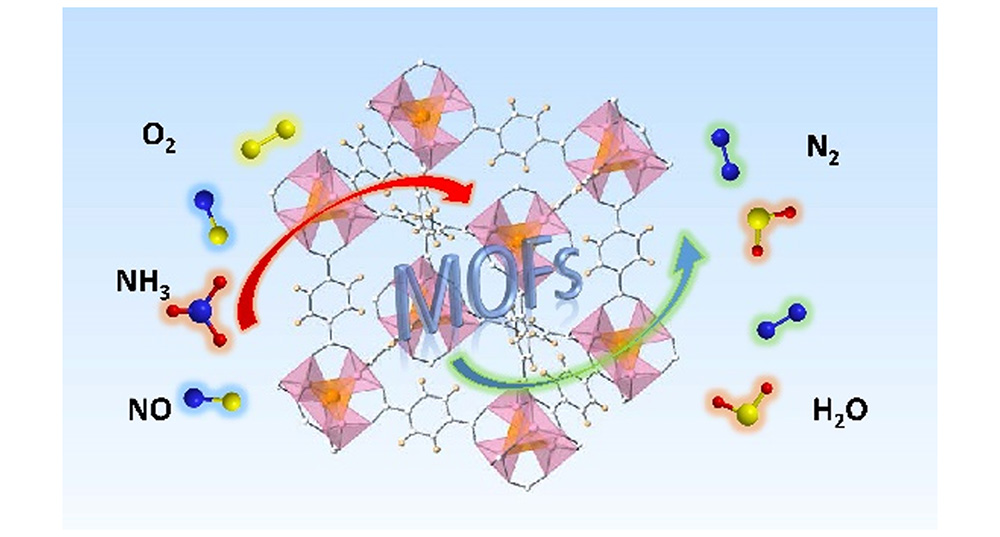
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |