随着液相激光熔蚀技术的发展,相关研究已从早期的基本原理探索和材料合成过程研究上升到新原理发现、新材料设计和新应用拓展。该文基于Web of Science数据库,采用文献计量学方法,分析了1998–2016年发表液相激光熔蚀技术研究的主要国家、发表文章总量、研究方向、研究机构、研究热点等相关信息,并总结了主要前沿研究内容与发展趋势。该文为广大科研工作者迅速了解和掌握液相激光熔蚀技术发展趋势和研究热点提供了相应的数据支持。
1960年代,梅曼(T. H. Maiman)在美国休斯公司实验室制成世界上第一台红宝石固态激光器[1],自此,激光技术广泛应用于科学研究与工业生产的众多领域。从1965年开始,人们发现在真空或者特定气氛条件下,激光技术可用于制备薄膜,于是脉冲激光沉积(pulsed laser deposition, PLD)技术开始兴起[2],预示着激光技术作为一种材料制备手段正式进入人们的视野。PLD技术利用激光的高能量将目标靶材瞬间气化,进而形成等离子体羽,最终在固定基底上沉积成膜。通过这种方法,研究者成功制备了一系列高品质的多晶薄膜,如陶瓷氧化物、氮化物、金属多层膜等[3]。相对于分子束外延、化学气相沉积等方法,PLD技术的工作成本要低得多,因而受到广泛重视。直到1990年代,纳米技术的兴起开启了微观材料合成设计研究的新大门。在时代潮流引领下,1993年,Henglein和Cotton分别尝试在水和不同溶剂中利用脉冲激光熔蚀金属靶材,均成功获得胶体纳米溶液[4,5]。至此,人们意识到,通过脉冲激光在液相体系下熔蚀目标靶材能够获得相应的纳米材料。由于熔蚀过程同时包括物理与化学变化,涉及复杂的热力学和动力学过程,最终产物也区别于普通化学法制备的同种材料,这种新奇之处使得液相激光熔蚀(laser ablation in liquids, LAL)技术正式登上纳米材料制备技术领域的大舞台。
通常情况下,LAL技术要求激光必须在液体环境下辐照目标靶材(见图1),通过“热作用”导致靶材料的气化或蒸发,并在靶与液体的界面,即液-固界面,产生一个等离子体羽,由于被液体包围,等离子体羽的膨胀趋势受到束缚,从而在等离子体羽内部产生一个很大的附加压强,最终形成一个高温高压高密度的极端区域[6]。在这种条件下,经激光熔蚀而脱离靶材的物质有可能相互反应,也有可能与液体中的成分反应,还可能与液体分子在界面处反应,极易产生处于亚稳状态的纳米材料[6]。根据激光参数(波长、脉宽等)不同,LAL过程中的作用机制与产物状态都会有很大差异,即使激光参数不变化,改变液体环境或者外场条件都可能导致新的现象出现。经过前期的知识积累和技术发展,LAL制备方法已经处于科学原理突破与重大技术创新的关键阶段。值得关注的是,我国不仅是国际上LAL纳米制备技术的主要开拓者之一,并且已在该领域的多个方面处于世界领先地位。尤其是近几年,随着综合国力的提升,我国也在加大对该技术领域的扶持力度。然而,国内关于这一技术领域的最新发展状态却鲜有报道。
本研究根据文献计量学的统计方法,依托Web of Science数据平台,对国内外LAL技术的发展动态进行归纳总结,客观定量地展现相关学科发展的整体布局与发展趋势,以期为国内科研工作者提供数据参考,促进LAL技术在纳米材料制备领域的快速发展。
本研究采用Web of Science数据库核心合集作为分析数据源,基于SCI-Expanded数据库,检索LAL技术领域的相关文献,并选择出版时间在1998年1月至2016年12月的文献作为分析数据,最终通过Web of Science数据库自带的引证报告进行分析,部分数据项用Excel、Origin等工具进行分析。需要说明的是,一方面,由于科学研究的出发点不同,很多研究者在文献中可能没有采用“laser ablation in liquids”作为技术称谓,而是采用“laser irradiation”、“laser fragmentation”、“laser generation”等其他术语;另一方面,“laser ablation”也可能涉及激光在电感耦合等离子体质谱(inductively coupled plasma mass spectrometry,ICP-MS)等技术当中的应用,又或与脉冲激光沉积(pulsed laser deposition, PLD)等技术具有十分接近的术语表达,而这些并非本研究所关注的内容,因此,在检索过程中,需要设置相当严格的条件限制,否则极有可能遗漏本技术领域的研究工作,也可能将其他技术领域的研究工作纳入本研究领域,使得检索结果的统计数据偏离实际。表1列出了本研究所采用的检索词条。
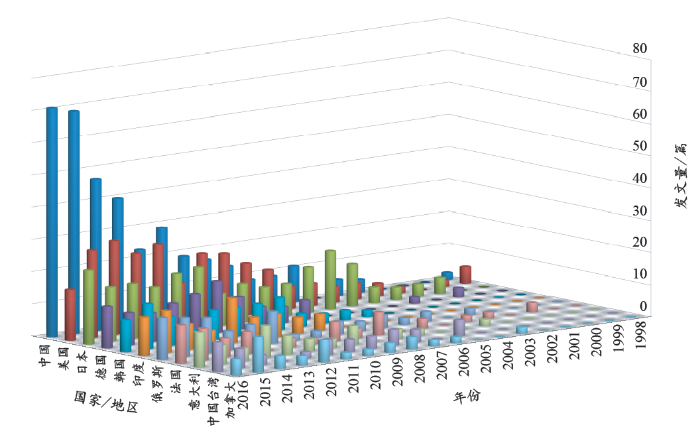
按照上述方法进行检索,1998年至2016年,涉及激光技术的液相体系下纳米材料合成及相关机理、应用研究的学术论文发文量为5 622篇,经过排除某些十分接近的技术领域的文献,LAL技术领域的实际发文量为1 554篇。按照Web of Science数据库自带的引证报告的分析结果,我们首先分析了19年来(1998–2016)各国发文总量的变化趋势。图2列出了排名前11位的国家或地区及其发文总量情况。按照引证报告的统计结果,中国是发文总量最高的国家,高达370篇,美国、日本以及德国分别位列第2、第3和第4位。而根据Barcikowski研究小组2009年的统计结果[7],日本和欧盟的发文量都高于中国,美国位列第4。这种情况说明,近年来中国在LAL技术领域的论文产出不断提高。图3进一步分析了以上国家或地区的发文量年度涨落情况,2003年以前,主要发文国家为美国、日本、德国、法国和意大利等,说明这些国家在LAL技术领域的研究起步较早。自2003年开始,中国、美国、日本、德国、韩国、印度、俄罗斯、法国、意大利和加拿大等国家或地区的论文产出都在逐步提高,说明该技术领域的研究工作越来越受到重视。值得注意的是,2011年以来,中国的发文量与美国、日本、德国等研究最为活跃的国家逐步接近,到2015年,已经超过其他国家或地区,成为年度发文量排名第一的国家。
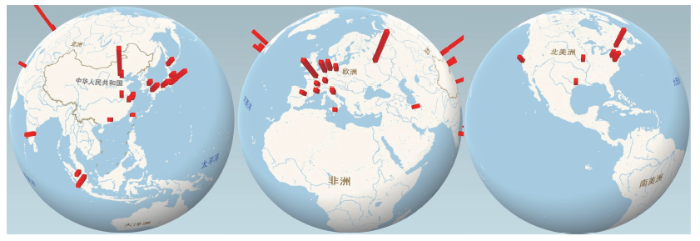
根据对检索文献所属研究机构的统计,我们列出了发文量排名前50的研究机构名单(见表2)及其地理分布情况(见图4)。这50个研究机构的发文量共为906篇,占全部发文量的57.85%,说明其主导了LAL技术领域的研究工作。我们注意到,由于Web of Science数据库自带的引证报告将每篇文献所涉及的全部研究机构都进行了统计,而部分研究机构在论文工作中可能只是合作单位,而非第一单位,因此,尽管图2中某些国家(如俄罗斯、意大利等)的发文量并不太高,但其研究机构依然具有较高的发文量,说明这些研究机构在相关领域的国际合作和交流活动中发挥重要作用。在图4中,我们根据地理区域的不同,对以上研究机构的分布进行了统计分析。在全球范围内,关于LAL技术各项研究的主要活跃区域集中在中国、东南亚、欧洲以及北美洲。在中国,从事相关研究的研究机构主要是中国科学院以及某些沿海省、市的高校(如,中山大学、浙江大学和天津大学等)。在东南亚地区,日本和新加坡的高校及科研院所一直引领该区域内的相关研究活动。在欧洲,德国和法国的研究活跃度要明显强于其他国家。在北美洲,主要是美国的政府机构(国防部、能源部等)对相关领域的研究表现出较大的积极性。
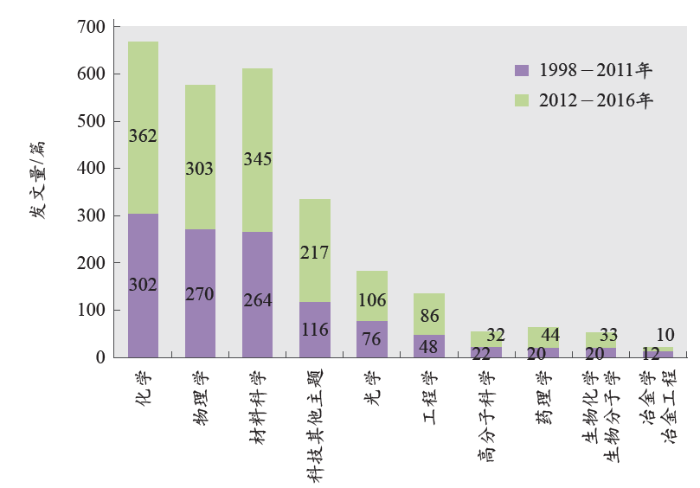
按照Web of Science数据库的“分析检索结果”功能中的“研究方向”选项,我们对所有文献的研究方向进行统计分析,并在图5中列出了排名前10的结果。分析表明,LAL技术领域的研究主要集中在化学、物理学和材料科学3个方向。涉及这3个方向的论文数量明显高于其他研究方向,甚至高出一个数量级。另外,经过分析发现,2012到2016年的5年里,各研究方向的发文量都明显高于前14年(1998–2011)的发文总量。该项结果表明,近几年,LAL技术促进社会发展的巨大潜力越来越受到国际社会的认可,所受到的重视程度也明显提升,相关科学研究更加活跃。值得一提的是,该项技术在生物医药领域的发展速度不可小觑。由于Web of Science数据库将生物医药领域划分为生物化学与生物分子学、药理学、细胞生物学、生物技术与应用微生物学、生物物理学、遗传学、毒理学、免疫学等众多细小的研究方向,所以在图5中并未给出整个生物医药领域的发文量信息。经过进一步统计,得到整个生物医药领域的发文总量为214篇,所占比例为13.77%。根据Barcikowski研究小组[7]2009年的统计结果,生物医药领域发文量所占比例只有1%左右,这说明LAL技术制备或改进的纳米材料在生物医药领域的应用研究越来越受到重视。
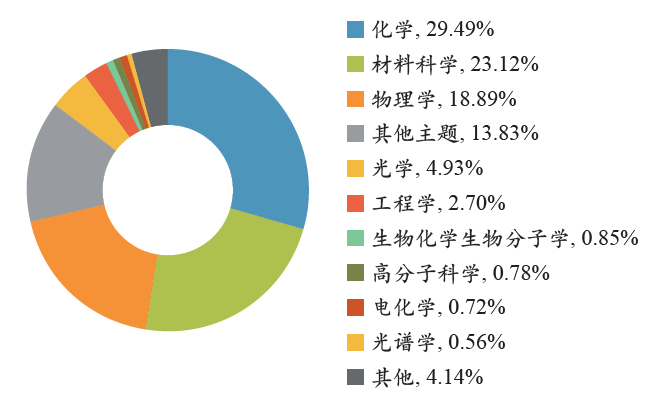
研究者一般使用论文被引用数据来评估学术论文和期刊的影响力,并获得相关的研究热点和发展趋势等相关信息。为了分析LAL技术领域的研究热点,我们在表3中列出了被引频次最高的15篇文献及其相关信息(包括发表期刊、发表年份、被引频次等)。分析表明,最受关注的研究材料为贵金属(Au、Ag等)体系,少数文献关注的是过渡族金属氧化物材料(CuO、ZnO等)体系。研究内容主要集中在3个方面:(1)胶体纳米颗粒的形成过程及尺寸、形貌调控研究;(2)胶体纳米颗粒的表面活性及反应性研究;(3)胶体纳米材料的性能应用研究。这些文献平均每年至少被引用13次,最高可达135次,说明它们自发表以来一直受到普遍关注。为了进一步分析目前的研究趋势,按照Web of Science数据库自带的“研究方向”分类,我们对这15篇文献的全部施引文献的研究方向进行统计分析,并在图6中列出了排名前10的研究方向。分析表明,尽管化学、材料科学、物理学依然是主要研究方向,但与图5相比,材料科学方向的文献比例已经超过物理学方向,说明在施引文献中,相关研究工作更加集中在化学和材料科学方面,引用者可能更加关注LAL技术的材料合成策略及其所涉及的化学过程。除此之外,电化学和光谱学方向的研究工作也明显增多,这也预示着LAL技术制备的胶体纳米材料在其他相关领域研究的进一步拓展。
尽管我们根据被引频次最高的15篇文献(表3)简要分析了液相激光熔蚀技术领域的热门研究内容,但仍不足以总结整个领域内的研究状况。通过分析检索文献的摘要内容,我们发现,对LAL技术在不同条件下制备纳米材料的过程调控、机制分析及材料性能应用的相关研究一直是研究者们关注的核心问题。因此,根据检索得到的文献,我们进一步总结得到LAL技术领域的主要热门研究内容,并归纳为“研究方法”、 “材料体系”和“应用方向”3个方面(见图7)。
由于在液相体系下激光的熔蚀过程决定了所得纳米材料的尺寸、形貌、成分组成等,而它们不仅可以间接反馈形成机制相关信息,还与材料性能及应用潜力直接关联,所以,采用不同的方法探索激光熔蚀过程,进而实现相关纳米材料的形成机制总结、合成过程调控、应用性能改进是LAL技术领域最受关注的研究内容之一。对于早期的研究方法,人们主要关注的是如何调节激光波长、脉冲频率、能量密度等相关参数,从而在液相体系下获得特殊的纳米材料并总结分析相应的形成机制[8,9],这部分研究工作所使用的方法也就是最为常见的“熔蚀靶材”(见图7)。前期研究表明,激光脉宽可以分为3类:超短脉宽激光(超快激光,即飞秒激光)、短脉宽激光(纳秒激光)、长脉宽激光(毫秒激光),而且这3类脉冲激光的熔蚀过程有着本质的不同[6]。在此基础上,新的研究方法也在不断涌现,如对盐溶液、胶体等直接进行“辐照溶液”[10,11,12,13],又或在电场/磁场下进行“外场辅助”的激光熔蚀[14,15,16]。这两种研究方法都为纳米材料形貌、尺寸、组成等各项参数的精确调控提供了契机。除以上研究方法之外,还有两种方法也受到普遍关注,即“溶剂诱导”[17,18,19]和“后续加工”[20,22]。前者通过改变溶剂种类调控胶体纳米颗粒的表面性质及生长过程,为胶体界面性质研究提供新的分析思路;后者主要通过水热、旋涂等途径将胶体纳米材料应用到不同方向,有利于进一步拓宽胶体纳米颗粒的应用领域,如生物传感、能源催化等。采用以上方法调控胶体纳米材料的形貌、尺寸及性能极大丰富了LAL技术的研究范畴。
研究者们也十分关注不同材料在LAL条件下组分、结构等变化规律的总结,这部分研究内容更侧重于材料本身各项参数演变。研究最为广泛的材料体系为“贵金属”、“半导体”和“磁性材料”(见图7)。对于“贵金属”体系而言,最具代表性的是Au、Ag、Pt及其合金。前期的研究表明,贵金属反应活性低,经激光熔蚀之后很难与液相或气相介质进行反应[23,24,25],因此,相关工作主要集中在观察胶体纳米颗粒的尺寸、形貌、组成等在激光参数和溶剂种类等条件变化时的演变规律。近年来,由于LAL技术制备的胶体纳米材料越来越凸显出绿色、高活性等优势,如何在贵金属纳米颗粒表面嫁接各种生物分子或与其他材料进行复合以实现多功能、高效能型的新材料逐渐成为研究最为集中的课题[26,27,28]。对于“半导体”体系,涵盖范围十分广泛,如氧化物(ZnO[29]、CuO[30]、TiO2[31]、SnO2[21]等)、硫化物(PbS[32]等)、硒化物(ZnSe[33]、CdSe[34]等)、氮化物(InN[35]、GaN[36]、GaAs[37]等)、碳化物(SiC[38]等)。对于这些材料,研究重点在于如何构造不同微观结构或形成复合体系,以提升它们在光学、电学、化学等各方面的性能,例如,SnO2纳米颗粒与石墨烯复合之后的导电性能[21]等。另一种材料体系是“磁性材料”,主要包括Co[39]、Ni[40]等金属,Mn3O4[41]、FeOx[42]等氧化物以及FeNi[43]、AuCo[44]等合金材料。研究者们一方面关注这类材料磁学性能与结构尺寸的关联,另一方面也十分在意是否能够通过磁性来辅助控制合成新材料和发现新机制,并拓宽相关材料的应用范畴。通过以上分析可以发现,只要能够以固态靶材形式存在的材料,几乎都可以利用LAL技术来合成相应的胶体纳米材料,并极有可能出现不同于常规方法制备的材料的新现象,充分体现了该技术的应用普适性。
自1990年代以来,LAL技术就被引入到纳米材料的制备策略当中。经过多年的发展,研究者已经就该技术能够成为一种制备高活性、绿色纳米材料的有效手段达成共识,而能否推进相关材料在各领域的应用发展也是目前最为火热的研究内容之一。针对“应用方向”方面的文献调研,我们通过所有检索文献总结出了3个主要的应用领域,即“生物医药”、“能源催化”和“环境保护”(见图7)。在“生物医药”领域,纳米材料的毒理作用一直备受关注。通常采用化学法制备的纳米材料表面带有活性剂或其他合成前驱体的残留成分,导致难以直接说明纳米材料本身的毒性情况,成为纳米材料在“生物医药”领域应用研究的首要障碍。然而,LAL技术制备的纳米材料无需添加任何表面活性剂或者反应前驱体,能够从根本上解决上述困扰,因此,通过该技术获得的纳米材料在“毒理分析”方面的研究十分活跃[45,46]。研究表明,通过激光在含有生物分子的溶液中直接熔蚀金属靶材(如,金等)能够一步实现胶体纳米材料与生物分子的偶联[47],也可以先制备胶体,再在其表面修饰生物分子[48]。因此,研究者可以在不与其他物质接触的前提下,设计得到具有多种功能的高活性生物材料,在“生物成像”、“生物传感”以及“生物制药”等方面都表现出具大的应用潜力[49,50,51,52]。LAL技术在“毒理分析”、“生物成像”、“生物传感”以及“生物制药”4个方面所发挥的积极作用使得该技术在“生物医药”领域的地位逐步上升。目前,在此领域,研究工作较为突出的课题组主要集中在国外,如德国的Barcikowski Stephan课题组、加拿大的Kabashin Andrei V.和Meunier Michel课题组等。
在“能源催化”领域,催化剂活性对于能量转化、物质分解、电子转移等各方面都十分关键。得益于胶体纳米材料处于亚稳态,且表面干净、性质活泼,LAL技术也开始在此领域崭露头角。现有研究表明,LAL技术制备的纳米催化剂(如,Au-CeO2、AuPt、PtCo、Pt-rGO等)相对于普通化学法制备的纳米催化剂具有更高的活性[53,54,55,56]。利用该技术获得的催化剂主要应用于3个方面:“光解水”、“电池”和“工业催化”。在“光解水”方面,中国科学院梁长浩课题组成功将该技术与能源研究相结合,他们通过LAL技术获取的胶体作为掺杂前驱体,在导电玻璃基底上制备的赤铁矿薄膜能够获得1.4 mA/cm2的光电流密度[57]。中山大学的杨国伟课题组通过LAL方法同时实现TiO2的还原以及与氧化石墨烯的复合,大大提高光解水制氢的效率[58]。天津大学的杜希文课题组基于LAL技术开发的高活性Co3O4催化剂的光解水产氧效率达到氧化钴材料的最高水平,甚至超过贵金属催化剂RuO2[59]。这些研究工作都发表在国际一流期刊,受到同行的广泛认可。在“电池”研究方面,已有报道证明:LAL技术制备的TiO2[60]、SnO2[61]等可以用作锂离子电池的阴极材料;Au胶体可以代替生物酶氧化葡萄糖分子进而实现生物燃料电池功能[62];在电泳技术辅助下,CuSe2胶体可以成膜并用于太阳能电池[63]。另外,在“工业催化”方面,主要的研究对象为甲醇、乙醇、甲酸等碳氢化合物[64,65,66]。值得一提的是,中国科学院的梁长浩课题组开发了一种高度分散的超细铂基催化剂,在甲醇氧化方面处于国际先进水平[56]。以上分析表明,在推进LAL技术应用于“能源催化”领域的研究工作当中,中国科学家已经开始进军世界前沿研究。
由于LAL技术制备的胶体能够用于检测和监视工业废水和汽车尾气的排放以及重金属和农药成分的残留,因此,其在“环境保护”方面的研究往往受到工业生产部门的热切关注。现有研究表明:该技术制备的胶体纳米材料能够广泛实现对重金属离子、食品和水中污染物(如黄嘌呤、病原体、苯肼等[67,68,69])以及易燃易爆气体(如CO、乙醇、HCl、H2等[70,71,72,73])的痕量检测。在这些研究当中,研究重点一方面在于如何提高纳米材料的选择性,另一方面在于如何实现同时检测和分辨多种污染物。LAL在相关领域的技术优势已经引起世界范围内研究者的重视。
值得一提的是,LAL技术之所以能够具有非常宽广的应用范畴和巨大的应用潜力,除了该技术能够提供一种简洁、绿色的合成手段之外,主要得益于它所提供的反应环境具有高温高压高密度的特征,其制备的胶体纳米颗粒往往具有亚稳态的物相或者结构,如果合理控制激光、溶剂等各项参数,很有可能形成具有特殊功能的纳米材料,而这恰好弥补了一般化学合成方法的局限[74]。因此,在LAL技术的应用研究中,如何基于亚稳态的特殊性来控制合成纳米材料的结构和性能也是相当重要的一个环节。例如,上面提到的中国科学院梁长浩课题组获得了高度分散的超细铂基催化剂,就是利用Mn胶体纳米颗粒的亚稳特性,成功将其负载在氧化石墨烯基底上,在此基础上,采用自牺牲式还原法在固定基底上成功合成超细铂基催化剂,从而获得了十分高效的催化性能[56];加拿大Guay小组在PEG溶液中熔蚀Au靶材,成功控制了亚稳态Au纳米颗粒的尺寸和形貌,使其具有优异的电子计算机断层扫描(CT)造影功能[48];中山大学的杨国伟课题组专门设计合成了亚稳态的金刚石、难熔合金及纳米晶等材料,大大拓展了相关材料的应用范畴[75,76,77]。这些研究表明,详细掌握亚稳态纳米材料的物性对于深入推进LAL技术的应用研究具有重要意义。
LAL技术在“生物医药”、“能源催化”、“环境保护”等拥有重大应用需求的领域中的应用潜力已经崭露头角。本研究从文献计量学角度对相关技术领域的发展趋势和研究热点内容进行了深入的分析。依托数据平台,总结了相关技术领域的主要发文国家、发文量、研究方向、研究机构、最受关注研究工作以及主要研究内容等多方面的最新信息,为加强广大科学工作者对相关领域的深入了解提供详实的数据支持,以期进一步推动LAL技术的发展。
The authors have declared that no competing interests exist.
| [1] |
DOI:10.1038/187493a0
URL
[Cite within: 1]
Schawlow and Townes 1 have proposed a technique for the generation of very monochromatic radiation in the infra-red optical region of the spectrum using an alkali vapour as the active medium. Javan 2 and Sanders 3 have discussed proposals involving electron-excited gaseous systems. In this laboratory an optical pumping technique has been successfully applied to a fluorescent solid resulting in the attainment of negative temperatures and stimulated optical emission at a wave-length of 6943 脜. ; the active material used was ruby (chromium in corundum).
|
| [2] |
|
| [3] |
DOI:10.1016/0022-3093(94)90285-2
URL
[Cite within: 1]
A comprehensive overview of what is required to set up and begin research in this newly developing technology and understand the basics of the process. Internationally recognized experts in their fields cover such fundamentals as history, theory, film characteristics, surface modification, laser technology, materials and applications including excellent reviews regarding the entire areas of semiconductor buffer layers, thin-film ferroelectrics and ferrites along with the work involving films deposited by PLD.
|
| [4] |
DOI:10.1155/2012/814745
URL
[Cite within: 1]
The optical properties of afterglow nanoparticles were successfully improved by the addition of polyethylene glycol (PEG) to an afterglow colloidal solution. Afterglow nanoparticles鈥 : , 鈥攚ere prepared by laser ablation in liquid. The quantum yields and the decay curves were measured by a fluorescence spectrophotometer. An increase in the amount of PEG added to the solution increased the quantum yield of the nanoparticles and improved the afterglow property in the initial portion of the decay curve. However, the afterglow property did not change after a substantial amount of time had passed. The afterglow nanoparticles were capped with PEG molecules, and surface defects of the nanoparticles were passivated, which decreased the optical properties. 1. Introduction In the past several years, there has been increasing interest in nanoparticles due to their unique properties and various applications in research fields, such as biotechnology and electronics. There are many methods available to create nanoparticles, such as gas-phase preparation [1, 2] and liquid-phase preparation [3, 4]. One of the liquid-phase preparation methods is laser ablation in liquid, which has recently been studied extensively [5鈥9]. While laser ablation in the gas phase, such as under vacuum and in argon, has been used for various research purposes [10], laser ablation, in liquid, has been investigated more recently [11, 12]. In the case of laser ablation in liquid, the target material in the liquid is irradiated with a focused, pulsed laser beam without using a chamber. Precipitation methods, such as the sol-gel method, are well known as synthesis methods for nanoparticles. However, it is difficult to prepare multielement nanoparticles with these techniques. In the case of the trioctylphosphine oxide (TOPO) method [4], the popular synthesis method used to create quantum dots, coating materials are limited to specific surfactants, such as TOPO. In contrast, in the case of laser ablation in liquid, multielement nanoparticles can be easily prepared. By using laser ablation in liquid, surfactant-free nanoparticles can be prepared, and/or a desirable coating material can be selected. We prepared functionalized multielement nanoparticles with afterglow properties by using laser ablation in liquid [13]. Afterglow is a promising optical property for various fields because of the long emitting time (e.g., several hours) after the excitation is blocked. In the case of normal fluorescence, materials emit during excitation but do not emit after the excitation is blocked. On the contrary, in
|
| [5] |
DOI:10.1366/0003702934066460
URL
[Cite within: 1]
Laser ablation of metals is a new and very powerful method for preparation of surface-enhanced Raman scattering (SERS) active colloids. The method is characterized by its simplicity and versatility. Stable Ag, Au, Pt, Pd, and Cu colloids are prepared by ablation of the metal for ~ 10 min in water and organic solvents. An important advantage of this approach over conventional chemical procedures is that the colloids are free of organic or ionic species. Consequently, the chemical and physical effects of ions or other adsorbates can be studied under carefully controlled conditions. The SERS activity of colloidal metals prepared by laser ablation is comparable or superior to that of chemically prepared colloids.
|
| [6] |
DOI:10.1002/chin.200738227
URL
[Cite within: 3]
Abstract ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 200 leading journals. To access a ChemInform Abstract, please click on HTML or PDF.
|
| [7] |
DOI:10.1007/s11051-009-9765-0
URL
[Cite within: 2]
ABSTRACT The number of publications on laser ablation and nanoparticle generation in liquids increased by the factor of 15 in the last decade, with comparable high impact of the most cited articles in this field. A nearly unlimited variety of nanoparticle material, liquid matrix, and conjugative agent can be combined to a huge variety of colloids within a few minutes of laser processing. However, this diversification makes it hard to identify main research directions without a comprehensive literature overview. This investigation evaluates the impact and structure of the literature in this field tagging most prolific subjects and articles. Using an optimized search algorithm, the data sets derived from Science Citation Index (1998鈥2008) allow for statements on publication subject clusters, impact of articles and journals, as well as mapping global spots of activities.
|
| [8] |
DOI:10.1021/jp047134+
URL
[Cite within: 1]
|
| [9] |
DOI:10.1039/b900654k
PMID:19440607
URL
[Cite within: 1]
Abstract In the past years, laser ablation synthesis in solution (LASiS) emerged as a reliable alternative to traditional chemical reduction methods for obtaining noble metal nanoparticles (NMNp). LASiS is a "green" technique for the synthesis of stable NMNp in water or in organic solvents, which does not need stabilizing molecules or other chemicals. The so obtained NMNp are highly available for further functionalization or can be used wherever unprotected metal nanoparticles are desired. Surface functionalization of NMNp can be monitored in real time by UV-visible spectroscopy of the plasmon resonance. However LASiS has some limitations in the size control of NMNp, which can be overcome by "chemical free" laser treatments of NMNp. In this paper we provide an overview of LASiS, size manipulation by laser irradiation and functionalization of NMNp, with special care in pointing out some of the main issues about this research area.
|
| [10] |
DOI:10.1021/jp027269k
URL
[Cite within: 1]
A simple, convenient, and general method for the synthesis of metal and metal alloy nanoparticles is presented. Irradiation of metal powders in suspension in either aqueous or organic solutions by unfocused 532 nm laser radiation produces nanoparticles with a homogeneous composition proportional to the composition of the starting metal powder mixture. This is demonstrated using UV61vis absorption spectroscopy, transmission electron microscopy, energy-dispersive X-ray spectroscopy, and modelization. The mechanism of alloy formation is discussed.
|
| [11] |
DOI:10.1021/j100566a003
URL
[Cite within: 1]
Abstract Aqueous and alcoholic solutions of silver perchlorate have been photolyzed by 253.7-nm light. The primary reaction of the photolysis is assumed to be electron transfer from solvent molecule to silver ion: (Ag+, ROH)cage + hv 鈫 (Ag, ROH+)cage. Variations of the mean quantum yield of silver atom formation with the concentrations of silver ion, hydrogen ion, and alcohols added are systematically interpreted by the concept that reactive species are surrounded by the solvent cage. It is proposed that the number of solvent molecules composing a cage would be about 10. The quantum yield increases with reaction temperature. Apparent activation energies of the quantum yields are about 2-6 kcal/mol.
|
| [12] |
DOI:10.1021/ja00347a011
URL
[Cite within: 1]
Reduction of HAuClhas been investigated pulse radiolytically in water and in water-in-oil microemulsions. Rate constants have been determined for Au+ e/sub aq/→ Au, 2Au→ Au+ Au, and Au+ R.→ Au+ R (where R. is an unidentified radical). On the longer time scale formation of colloidal gold, nAu→ (Au)n, has been observed. Rate of colloidal gold formation has also been studied in the bombardment of HAuClsolutions by 353-nm 60-mJ 3-5-ns laser pulses. Hydrodynamic diameters and polydispersities of empty and colloidal-gold-containing microemulsions have been determined by electron micrography. There are a number of advantages of forming colloidal particles in microemulsions. Under identical conditions a greater amount of colloidal particles is formed than that in water. Colloidal gold particles formed in microemulsions are smaller and more uniform than those obtained in homogeneous solutions
|
| [13] |
DOI:10.1021/jp0441588
URL
[Cite within: 1]
|
| [14] |
DOI:10.1021/cg800633j
URL
[Cite within: 1]
The high-pressure nanophase, that is, the metastable tetragonal structure, of germanium is trapped by a facile technique named electrical-field assisted pulsed laser ablation in liquid at ambient pressure and temperature. On the basis of X-ray diffraction, transmission electron microscopy, and Raman scattering analyses, the trapped Ge nanophase is identified to be the tetragonal structure rather than the diamond structure of bulk germanium. First-principles calculations are used to clarify the physical and chemical mechanisms of the tetragonal Ge formation upon laser ablation in liquid.
|
| [15] |
DOI:10.1021/jp802529r
URL
[Cite within: 1]
We have developed a unique technique, the facile electrical-field-assisted laser ablation in liquid (EFLAL) without any catalyst or organic additives, to controllably fabricate the mass production of GeO
|
| [16] |
DOI:10.1039/c5tc02160j
URL
[Cite within: 1]
An approach to assemble high aspect ratio nanostrands consisting of magnetic nanowires and their incorporation in a polymer with the aim of tailoring transparent FeNi nanostrand–PMMA-composites is presented. These nanostrands are controllable in length (<600 μm) and width (<12 μm)viaprocess parameters and have an ultra-high aspect ratio (65160). This rapid and universal method provides flexible and transparent magnetic materials with tunable transparency and magnetic attraction force by adjusting the density of nanoparticles in the composite. These composites can be used as a window coating for shielding radio frequency electromagnetic waves while being transparent in the optical range.
|
| [17] |
DOI:10.1038/srep32631
PMID:27599448
URL
[Cite within: 1]
Understanding the thermodynamic behavior and growth kinetics of colloidal nanoparticles (NPs) is essential to synthesize materials with desirable structures and properties. In this paper, we present specific uncapped Te colloidal NPs obtained through laser ablation of Te in various protic or aprotic solvents. At ambient temperature and pressure, the uncapped Te NPs spontaneously exhibited analogous evolution and growth of 鈥渘anoparticle-nanochain-agglomerate-microsphere鈥 in different solvents. The distinctive growth kinetics of the formation of nanochains strongly depended on the polarity and dielectric constant of solvent molecules. The growth rate of agglomerates and microspheres was closely related to the zeta potential of the colloidal solution of Te nanochains and the average size of Te agglomerates. Furthermore, the resulting uncapped Te NPs and Te nanochains displayed a prominent size-dependent and structure-inherited chemical reductive ability. These findings provide insights into the growth of active uncapped nanoparticles in various dispersion media. This study also provides an alternative route in designing novel nanostructures of alloys, telluride, and functional composites using Te as a unique reactive precursor.
|
| [18] |
DOI:10.1016/j.apsusc.2004.09.065
URL
[Cite within: 1]
Laser ablation of Co, CoO and Co 3 O 4 was carried out in water and hexane. Nano-sized particles were produced from all materials in both solvents. It was found that atomic compositions of nanoparticles depended on solvent species in which laser ablation was carried out. In water, Co 3 O 4 nanoparticles were produced from all materials. In hexane, Co nanoparticles were produced from Co 3 O 4 and Co, while CoO particles were dominantly produced from CoO.
|
| [19] |
DOI:10.1021/la0637061
PMID:17489616
URL
[Cite within: 1]
Stable colloidal solutions of free silver nanoparticles (AgNPs) have been synthesized without reducing and stabilizing agents in pure acetonitrile and N,N-dimethylformamide by laser ablation of the bulk metal. Synthesis in tetrahydrofuran and dimethyl sulfoxide gave nanoparticles surrounded by a carbon shell or included in a carbon matrix. Mie theory for free and core@shell spheres accounts for the UV-vis spectra of the nanoparticles and allows their structural characterization. Transmission electron microscopy confirms the structure of the synthesized AgNPs. It is shown that free nanoparticles can be immediately functionalized, without further treatments, in the organic solvent used for the synthesis with molecules which are soluble in the same solvent.
|
| [20] |
DOI:10.1039/c3cp53307g
PMID:24162361
URL
[Cite within: 1]
We report a self-sacrificed in situ growth design toward preparation of ZnTiO3-TiO2 heterojunction structure. Highly reactive zinc oxide colloidal particles derived by laser ablation in liquids can react with TiO2 nanotubes to form a lamellar ZnTiO3 nanosheet structure in a hydrothermal-treatment process. Such hybrid structural product was characterized by X-ray diffraction, scanning and transmission electron microscopy, UV-vis diffuse reflection spectroscopy and X-ray photoelectron spectroscopy. The enhanced photocatalytic activity of the hybrid structure toward degradation of methyl orange (MO) and pentachlorophenol (PCP) molecules was demonstrated and compared with single phase TiO2, as a result of the efficient separation of light excited electrons and holes at the hetero-interfaces in the two semiconductors.
|
| [21] |
DOI:10.1039/c4cp00554f
PMID:24699526
URL
[Cite within: 2]
Quantum-sized SnO2nanocrystals can be well dispersed on reduced graphene oxide (rGO) nanosheets through a convenient one-potin situreduction route without using any other chemical reagent or source. Highly reactive metastable tin oxide (SnOx) nanoparticles (NPs) were used as reducing agents and composite precursors derived by the laser ablation in liquid (LAL) technique. Moreover, the growth and phase transition of LAL-induced SnOxNPs and graphene oxide (GO) were examined by optical absorption, X-ray diffraction, X-ray photoelectron spectroscopy, Raman spectroscopy and high-resolution transmission electron microscopy. Highly dispersed SnOxNPs can also prevent rGO from being restacked into a multilayer structure during GO reduction. Given the good electron transfer ability and unsaturated dangling bonds of rGO, as well as the ample electrocatalytic active sites of quantum-sized SnO2NPs on unfolded rGO sheets, the fabricated SnO2鈥搑GO nanocomposite exhibited excellent performance in the non-enzymatic electrochemical detection of glucose molecules. The use of LAL-induced reactive NPs forin situGO reduction is also expected to be a universal and environmentally friendly approach for the formation of various rGO-based nanocomposites.
|
| [22] |
DOI:10.1039/c5ra05376e
URL
[Cite within: 1]
In this paper, we report a rational sandwich composite structure consisting of polyaniline (PANI), amorphous TiO2(a-TiO2), and a GO network as an anode material for lithium-ion batteries (LIBs). After the synthesis of the a-TiO2–GO composite assisted by laser ablation in liquid, PANI nanorods are vertically grown on the both sides of a-TiO2–GO nanosheets to obtain a stable sandwich structure. The morphology and components of the composites are confirmed by scanning electron microscopy, transmission electron microscopy, and Raman spectroscopy. As a typical anode material in LIBs, the fabricated sandwich composites display a high rate capability and long cycle life. A first discharge capacity of 1335 mA h g611is shown at 50 mA g611and a reversible capacity of 435 mA h g611is achieved after 250 cycles at 100 mA g611. Even at a high cycling rate of 10 A g611, the sandwich products exhibit a stable capacity of 141 mA h g611. This effort highlights the design of a sandwich structure using amorphous TiO2, GO, and PANI nanorods and its potential benefits for LIB application.
|
| [23] |
DOI:10.1021/jp110907d
URL
[Cite within: 1]
Laser ablation (LA) of a Ag target in aqueous solutions of some strongly adsorbing ions resulted in formation of chemically modified Ag nanoparticles (NPs). A prospective development of this approach into a one-pot synthesis of hybrid Ag NPs鈭抩rganic species systems is conditioned by assessment of the factors affecting the hybrid system formation and stability during LA. In this study, intermittent LA of a Ag target accompanied by fragmentation of growing Ag NPs was carried out in aqueous solutions of 2,2鈥-bipyridine and/or of a cationic free-base porphyrin with nanosecond laser pulses of 1064 and 532 nm wavelengths. Ag NP/organic adsorbate systems resulting from each of the individual stages of the LA process were probed by SERS (surface-enhanced Raman scattering) and SPE (surface plasmon extinction) spectral measurements. The morphologies of selected systems were visualized by TEM (transmission electron microscopy). The efficiency of Ag NP fragmentation during LA (which corresponds to the efficiency of laser pulse absorption by the NPs) and the rate of the organic molecules' thermal desorption from the surfaces of Ag NPs heated by the laser pulse absorption have been recognized as crucial factors affecting the hybrid system stability.
|
| [24] |
DOI:10.1021/jp076680a
URL
[Cite within: 1]
Silver nanoparticle hydrosol formation by laser ablation (LA) of a Ag target immersed in pure water and in aqueous electrolyte solutions (HCl, NaCl, NaOH, AgNO3, and/or Na2S2O3) of various concentrations has been followed by surface plasmon extinction (SPE) spectral measurements and, for selected samples, by transmission electron microscopy (TEM), scanning electron microscopy (SEM) imaging, and energy dispersive X-ray (EDX) analysis. The laser ablation process accompanied by the Ag nanoparticle fragmentation (NF) has been performed with nanosecond laser pulses, by employing either a continuous or an intermittent irradiation regime. SPE spectra have been recorded either after each irradiation step of the stepwise procedure or at the end of the continuous one and, additionally, during a subsequent aging of Ag hydrosols. The presence of HCl, NaCl, and/or NaOH during LA/NF has led to the stabilization of the resulting Ag nanoparticles, while the presence of AgNO3 and Na2S2O3 has shown a destabilizing effect. ...
|
| [25] |
DOI:10.1021/la0637061
PMID:17489616
URL
[Cite within: 1]
Stable colloidal solutions of free silver nanoparticles (AgNPs) have been synthesized without reducing and stabilizing agents in pure acetonitrile and N,N-dimethylformamide by laser ablation of the bulk metal. Synthesis in tetrahydrofuran and dimethyl sulfoxide gave nanoparticles surrounded by a carbon shell or included in a carbon matrix. Mie theory for free and core@shell spheres accounts for the UV-vis spectra of the nanoparticles and allows their structural characterization. Transmission electron microscopy confirms the structure of the synthesized AgNPs. It is shown that free nanoparticles can be immediately functionalized, without further treatments, in the organic solvent used for the synthesis with molecules which are soluble in the same solvent.
|
| [26] |
DOI:10.1002/adfm.200801526
URL
[Cite within: 1]
Abstract A single step approach to tailored nanoparticle-bioconjugates, enabling the generation of gold nanoparticles by laser ablation and their in situ conjugation with any biomolecule bearing an electron donating function (e.g., thiolated oligonucleotides) is established. The integrity of oligonucleotides after conjugation and the stability of bioconjugates in physiological media are investigated. Their size is tailorable via process parameters. This rapid and universal method may provide biochemists with various nanoparticle-bioconjugates for screening the often unpredictable structure鈥揻unction relationship.
|
| [27] |
DOI:10.1186/1477-3155-8-21
PMID:20731831
URL
[Cite within: 1]
Background Bio-conjugated nanoparticles are important analytical tools with emerging biological and medical applications. In this context, in situconjugation of nanoparticles with biomolecules via...
|
| [28] |
DOI:10.1021/jp501489t
URL
[Cite within: 1]
Gold nanoparticles (AuNPs) covalently bound to biomolecules, termed bioconjugates,1 are highly relevant for biological applications like drug targeting or bioimaging. Here, the net charge of the bioconjugate is one key parameter affecting biocompatibility and cell membrane interaction. However, when negatively charged AuNPs are conjugated to positively charged biomolecules, resulting charge compensation compromises the stability of the conjugates. In this work, laser-generated negatively charged AuNPs exhibiting a bare surface were used as a model and separately conjugated to cell penetrating peptides (CPPs) carrying different positive net charges. Occurring charge compensation leads to two regimes where stable bioconjugates are obtained on both sides of the bioconjugate’s isoelectric point. These regimes can be controlled by the peptide’s net charge. Generally, increasing the peptide’s net charges yields stable positively charged bioconjugates with decreased surface coverages. To demonstrate the compatib...
|
| [29] |
DOI:10.1021/jp046747j
PMID:16850993
URL
[Cite within: 1]
ZnO nanoparticles were prepared by laser ablation of a zinc metal plate in a liquid environment using different surfactant (cationic, anionic, amphoteric, and nonionic) solutions. The nanoparticles were obtained in deionized water and in all surfactant solutions except the anionic surfactant solution. The average particle size and the standard deviation of particle size decreased with increasing amphoteric and nonionic surfactant concentrations. With the increase of the amphoteric surfactant concentration, the intensity of the defect emission caused by oxygen vacancies of ZnO rapidly decreased, while the exciton emission intensity increased. This indicates that anionic oxygen in the amphoteric surfactant molecules effectively occupied the oxygen vacancy sites at the ZnO nanoparticle surface due to charge matching with the positively charged ZnO nanoparticles.
|
| [30] |
DOI:10.1021/jp907237q
URL
[Cite within: 1]
In the past decade, it has become possible to synthesize many classes of nanoscale building blocks with controlled structure, size, and shape for applications in electronics, photonics, chemical engineering, medicine, etc. Successively, researchers need face a fundamental challenge: how to use nanoscale blocks to build functional structures or devices. Nanomanufacturing, fabricating, or assembling of building blocks for large-scale applications thus urgently emerges in the presented nanotechnology. For this issue, in this paper we report a unique nanomanufacturing, which can achieve from nanocrystal synthesis to sequential functional structure nanomanufacturing within one step. This novel technique is the electrical field assisted laser ablation in liquid (EFLAL). Using EFLAL, we first synthesize CuO nanocrystals and sequentially fabricate CuO functional structures, i.e., nanospindles with shape-dependent optical absorption that are expected to be applicable to biology or medicine. Importantly, these two ...
|
| [31] |
DOI:10.1166/jnn.2009.1114
PMID:19928229
URL
[Cite within: 1]
Synthesis of colloidal solution of TiO2 Nanoparticles by pulsed laser ablation in water, without any surfactant is studied. Fundamental wavelength of Nd:YAG laser operating at 35 mJ/pulse energy, 10 ns pulse width and 10 Hz repetition rate is used for the ablation of titanium target placed in water. Laser ablation of Titanium target in the water produces violet colored, highly stable colloidal solution for months even in the absence of any capping agents. UV-Visible absorption, Photoluminescence, X-ray diffraction, SEM and FTIR spectroscopic technique are used for the characterization of produced Nanomaterials. Six emission bands are observed in the photoluminescence spectrum of the produced sample. Origin of all the lines in the PL spectrum has been discussed. Possible reaction mechanism for the laser matter interaction and synthesis of nanomaterial are discussed.
|
| [32] |
DOI:10.1038/ncomms2637
PMID:23591862
URL
[Cite within: 1]
Monodisperse colloidal with size dispersions <10% are of great importance in realizing functionality manipulation, as well as building advanced devices, and have been normally synthesized via 'bottom-up' colloidal chemistry. Here we report a facile and environmentally friendly 'top-down' strategy towards highly crystalline monodisperse colloidal PbS with controllable sizes and narrow dispersions 5.5%<蟽<9.1%, based on laser irradiation of a suspension of polydisperse PbS with larger sizes. The colloidal demonstrate size-tunable near-infrared photoluminescence, and self-assemble into well-ordered two-dimensional or three-dimensional superlattices due to the small degree of polydispersity and surface capping of 1-dodecanethiol, not only serving as a but also a sulphur source. The acquisition of monodisperse colloidal PbS is ascribed to both the quantum-confinement effect of and the size-selective-vaporization effect of the millisecond pulse laser with monochromaticity and low intensity.
|
| [33] |
DOI:10.1007/s10876-013-0589-9
URL
[Cite within: 1]
Synthesis of the zinc selenide (ZnSe) nanocrystals (NCs) by pulsed laser ablation approach is reported in two distinct liquid media (ethanol and acetone) by means of the 1st harmonic of high frequency Nd:YAG laser. Based on the X-ray diffraction (XRD) pattern, ZnSe NCs have both wurtzite and zinc blende structures with some overlapping peaks. Transmission electron microscopy (TEM) reveals that as-synthesized NCs are relatively monodispersed and spherical in shape. UV–Vis spectra indicate that the band gap of ZnSe NCs in acetone and ethanol is blue shifted comparing to the band gap of bulk ZnSe which is due to quantum confinement effect. According to the XRD results, TEM observations and UV–Vis spectroscopy, as-synthesized ZnSe NCs in ethanol are larger than the ones in acetone. Two kinds of band edge and deep level emissions are identified by means of the room temperature photoluminescence spectroscopy.
|
| [34] |
|
| [35] |
DOI:10.1088/2040-8978/17/10/105903
URL
[Cite within: 1]
In this work, we present a hybrid indium nitride particle/polycrystalline silicon solar cell based on 230 nm size indium nitride particles (InN-Ps) obtained through laser ablation. The solar cell performance measurements indicate that there is an absolute 1.5% increase (Δ) in the overall solar cell efficiency due to the presence of InN-Ps. Within the spectral range 300–1100 nm, improvements of up to 8.26% are observed in the external quantum efficiency (EQE) and increases of up to 8.75% are observed in the internal quantum efficiency (IQE) values of the corresponding solar cell. The enhancement in power performance is due to the down-shifting properties of the InN-Ps. The electrical measurements are supplemented by TEM, Raman, UV/VIS and PL spectroscopy of the InN-Ps.
|
| [36] |
DOI:10.1007/s11051-016-3440-z
URL
[Cite within: 1]
We demonstrate the synthesis of GaN nanocrystals (NCs) with the sizes of less than the doubled exciton Bohr radius leading quantum confinement effects via a single-step technique. The generation of colloidal GaN nanoparticles (NPs) in organic solution through nanosecond (ns) and femtosecond (fs) pulsed laser ablation (PLA) of GaN powder was carried out. Ns PLA in ethanol and polymer matrix resulted in amorphous GaN-NPs with the size distribution of 12.4 卤 7.0 and 6.4 卤 2.3 nm, respectively, whereas fs PLA in ethanol produced colloidal GaN-NCs with spherical shape within 4.2 卤 1.9 nm particle size distribution. XRD and selected area electron diffraction analysis of the product via fs PLA revealed that GaN-NCs are in wurtzite structure. Moreover, X-ray photoelectron spectroscopy measurements also confirm the presence of GaN nanomaterials. The colloidal GaN-NCs solution exhibits strong blue shift in the absorption spectrum compared to that of the GaN-NPs via ns PLA in ethanol. Furthermore, the photoluminescence emission behavior of fs PLA-generated GaN-NCs in the 295-400 nm wavelength range is observed with a peak position located at 305 nm showing a strong blue shift with respect to the bulk GaN.
|
| [37] |
DOI:10.1016/j.apsusc.2005.03.004
URL
[Cite within: 1]
In this work colloidal quantum dots (QDs) of GaAs and CdS semiconductors have been formed by laser ablation in the liquid media. The pulsed passive mode-locked Nd:YAG laser at 106402nm wavelength with pulse duration τ imp 02=023302ps and energy 3002mJ was used. The luminescence of the colloidal QDs was excited by irradiation at 35502nm, the third harmonic of the Nd:YAG laser. The optical absorption and the photoluminescence spectra of the GaAs and CdS colloidal QDs have been investigated. The large blue shift of the photoluminescence, connected to size effects, was evaluated. The location of the maximum of luminescence spectra at the wavelengths 40502nm (CdS) and 42002nm (GaAs) give calculated sizes of QDs of 2–302nm.
|
| [38] |
DOI:10.1039/b909800c
URL
[Cite within: 1]
A simple and flexible route is presented for the fabrication of ultrafine β-SiC quantum dots (QDs) based on laser ablation of silicon wafers immersed in ethanol and subsequent etching. The obtained β-SiC QDs are nearly monodispersed and about 3.5 nm in size. The relative content of β-SiC after laser ablation depends on the liquid phase's ability to supply carbon atoms at a certain laser fluence. Proper liquid media with appropriate carbon atoms supply capacity can lead to nearly pure β-SiC in the as-prepared sample. The obtained β-SiC QDs exhibit strong and stable emission in the violet region, significantly blue-shifting relative to that of bulk SiC. This big blue shift of emission is attributed to the significant quantum confinement effect induced by their ultrafine size. This method can be extended to produce some other ultrafine Si compounds which are usually formed at high temperature and/or high pressure. This study could present the building blocks of nanostructured devices as violet light sources and new materials in biological molecular labels.
|
| [39] |
DOI:10.1016/j.matlet.2007.09.038
URL
[Cite within: 1]
Nanoparticles of nickel (Ni) and cobalt (Co) have been produced through laser ablation in organic solution. Pure Ni, Ni 50 Co 50 alloy, and Co metal plates as the targets, respectively, have been irradiated under 532nm Nd:YAG laser in ethylene glycol (EG). The produced metal nanoparticles are stabilized with poly ( N -vinyl-2-pyrrollidone) (PVP) in EG. Under the same laser fluence and irradiation time, the average particle size of Ni nanoparticles is 8nm in diameter, smaller than 22nm of Co nanoparticles. The laser-induced structural transition at interface of bulk metal and liquid has been investigated. It has been found that laser-generated Co nanoparticles show fcc structure, while the target of laser ablation is hexagonal Co bulk. In addition, the surface plasmon resonance (SPR) absorptions of Ni and Co nanoparticles stabled by PVP in EG are 355nm and 510nm, respectively. This research demonstrates that laser ablation in liquid, a one-step, non-catalyst process, can produce stable Ni and Co nanoparticles.
|
| [40] |
DOI:10.1039/c1jm12320c
URL
[Cite within: 1]
Dumbbell shaped nickel nanoparticles have been synthesized for the first time by laser induced fragmentation of bulk nickel powder in aqueous sodium dodecyl sulfate. The focused output of the third harmonic from a nanosecond pulsed Nd:YAG laser operating at 35 mJ/pulse energy was used for the photo-fragmentation of micron size particles suspended in 50 mM aqueous medium. Nickel anisotropic nanocrystals have been grown by recrystallisation of nickel particles due to laser plasma generated in sodium dodecyl sulfate solution. The Vibrating Sample Magnetometer measurements of dumbbell shaped nickel nanostructure show their good ferromagnetic nature. The mechanism of the synthesis of nanostructure through melting and fragmentation has been also discussed.
|
| [41] |
DOI:10.1021/jp104510a
URL
[Cite within: 1]
We present a clean synthesis of well-crystallized pure Mn3O4 nanoparticles by pulsed laser ablation of an Mn metal plate immersed in deionized water at room temperature. The particle size, phase structure, and magnetic properties are characterized by X-ray diffraction, transmission electron microscopy, Fourier transform infrared spectra, and superconducting quantum interference device. The colloids solution of Mn3O4 nanocrystals with narrow particles size distribution could be facile obtained in a single step without addition of any other chemical reagents. As-synthesized pure manganese oxide nanoparticles show good performance in significant and rapid removal of trace amounts of pentachlorophenol. Thoroughly distinct from the general chemical process for growing manganese oxide nanoparticles, here the formation of manganese oxide nanocrystalline is based on reactions between laser-generated manganese species and water molecules in a local high-temperature and high-pressure plume鈭抣iquid interface region.
|
| [42] |
DOI:10.1016/j.apsusc.2013.11.009
URL
[Cite within: 1]
Iron oxide (Fe2O3) bulk powder have been ablated/fragmented in different liquid medium by Nd:YAG laser beam using 1064nm wavelength. Sodium dodecyl sulfate (SDS), cetyltrimethyl ammonium bromide (CTAB) and double distilled water (DDW) are used as liquid medium. Crystalline size, lattice strain, phase and structure of ablated particles have been investigated using synchrotron X-ray diffraction. Optical band gap energy of as purchased Fe2O3 found 1.92eV that increased to 2.03eV after ablation in CTAB determined by UV–vis absorption spectroscopy. Magnetic properties have been analyzed by hysteresis loops using vibrating sample magnetometer (VSM). Crystalline sizes have been found in the range of 29.23–16.54nm and coercivity tailored in the range of 206.91–298.36Oe using laser ablation. Saturation magnetization and remanence have been found in the range of 0.013–3.41emu/g and 0.0023–.0.51emu/g respectively. Particle shape and size have been examined by scanning electron microscopy (SEM). CTAB (cationic) and SDS (anionic) surfactants are used as capping agent. CTAB produces phase transformation in ablated iron oxide (Fe2O3). Crystallinity and crystalline size of ablated particles in DDW increased due to presence of rich oxygen in it due to oxidation. Ablated Fe2O3 nanoparticles have been widely used experimentally for numerous in vivo applications such as MRI contrast enhancement agent, tissue repair, immunoassay, detoxification of biological fluids, hyperthermia, drug delivery and cell separation.
|
| [43] |
DOI:10.1039/c5tc02160j
URL
[Cite within: 1]
An approach to assemble high aspect ratio nanostrands consisting of magnetic nanowires and their incorporation in a polymer with the aim of tailoring transparent FeNi nanostrand–PMMA-composites is presented. These nanostrands are controllable in length (<600 μm) and width (<12 μm)viaprocess parameters and have an ultra-high aspect ratio (65160). This rapid and universal method provides flexible and transparent magnetic materials with tunable transparency and magnetic attraction force by adjusting the density of nanoparticles in the composite. These composites can be used as a window coating for shielding radio frequency electromagnetic waves while being transparent in the optical range.
|
| [44] |
DOI:10.1039/c3nr01119d
PMID:23685617
URL
[Cite within: 1]
We describe an environmentally friendly, top-down approach to the synthesis of Au89Fe11 nanoparticles (NPs). The plasmonic response of the gold moiety and the magnetism of the iron moiety coexist in the Au89Fe11 nanoalloy with strong modification compared to single element NPs, revealing a non-linear surface plasmon resonance dependence on the iron fraction and a transition from paramagnetic to a spin-glass state at low temperature. These nanoalloys are accessible to conjugation with thiolated molecules and they are promising contrast agents for magnetic resonance imaging.
|
| [45] |
DOI:10.3762/bjnano.6.66
PMID:25821705
URL
[Cite within: 1]
The use of engineered nanoparticles has risen exponentially over the last decade. Applications are manifold and include utilisation in industrial goods as well as medical and consumer products. Gold and silver nanoparticles play an important role in the current increase of nanoparticle usage. However, our understanding concerning possible side effects of this increased exposure to particles, which are frequently in the same size regime as medium sized biomolecules and accessorily possess highly active surfaces, is still incomplete. That particularly applies to reproductive aspects, were defects can be passed onto following generations. This review gives a brief overview of the most recent findings concerning reprotoxicological effects. The here presented data elucidate how composition, size and surface modification of nanoparticles influence viablility and functionality of reproduction relevant cells derived from various animal models. While in vitro cultured embryos displayed no toxic effects after the microinjection of gold and silver nanoparticles, sperm fertility parameters deteriorated after co-incubation with ligand free gold nanoparticles. However, the effect could be alleviated by bio-coating the nanoparticles, which even applies to silver and silver-rich alloy nanoparticles. The most sensitive test system appeared to be in vitro oocyte maturation showing a dose-dependent response towards protein (BSA) coated gold-silver alloy and silver nanoparticles leading up to complete arrest of maturation. Recent biodistribution studies confirmed that nanoparticles gain access to the ovaries and also penetrate the blood-testis and placental barrier. Thus, the design of nanoparticles with increased biosafety is highly relevant for biomedical applications.
|
| [46] |
DOI:10.1039/c3an01463k
PMID:24171189
URL
[Cite within: 1]
Abstract Metal and alloy nanoparticles are increasingly developed for biomedical applications, while a firm understanding of their biocompatibility is still missing. Various properties have been reported to influence the toxic potential of nanoparticles. This study aimed to assess the impact of nanoparticle size, surface ligands and chemical composition of gold, silver or gold-silver alloy nanoparticles on mammalian gametes. An in vitro assay for porcine gametes was developed, since these are delicate primary cells, for which well-established culture systems exist and functional parameters are defined. During coincubation with oocytes for 46 h neither any of the tested gold nanoparticles nor the gold-silver alloy particles with a silver molar fraction of up to 50% showed any impact on oocyte maturation. Alloy nanoparticles with 80% silver molar fraction and pure silver nanoparticles inhibited cumulus-oocyte maturation. Confocal microscopy revealed a selective uptake of gold nanoparticles by oocytes, while silver and alloy particles mainly accumulated in the cumulus cell layer surrounding the oocyte. Interestingly sperm vitality parameters (motility, membrane integrity and morphology) were not affected by any of the tested nanoparticles. Only sporadic association of nanoparticles with the sperm plasma membrane was found by transmission electron microscopy. In conclusion, mammalian oocytes were sensitive to silver containing nanoparticles. Likely, the delicate process of completing meiosis in maternal gametes features high vulnerability towards nanomaterial derived toxicity. The results imply that released Ag(+)-ions are responsible for the observed toxicity, but the compounding into an alloy seemed to alleviate the toxic effects to a certain extent.
|
| [47] |
DOI:10.1186/1477-3155-8-21
PMID:20731831
URL
[Cite within: 1]
Background Bio-conjugated nanoparticles are important analytical tools with emerging biological and medical applications. In this context, in situconjugation of nanoparticles with biomolecules via...
|
| [48] |
DOI:10.1039/C6TB01162D
URL
[Cite within: 2]
In recent years, pulsed laser ablation in liquids (PLAL) has emerged as a new green chemistry method to produce different types of nanoparticles (NPs). It does not require the use of reducing or stabilizing agents, therefore enabling the synthesis of NPs with highly-pure surfaces. In this study, pure Au NPs were produced by PLAL in aqueous solutions, sterically stabilized using minimal PEG excess, and functionalized with manganese chelates to produce a dual CT/MRI contrast agent. The small hydrodynamic size (36.5 nm), low polydispersity (0.2) and colloidal stability of Au NPs@PEG-Mn2+ were demonstrated by DLS. The particles were further characterized by TEM, XPS, FTIR and 1H NMR to confirm the purity of the Au surfaces (i.e. free from the common residual chemicals found after NP synthesis) and the presence of the different surface molecules. The potential of these particles as contrast agents for CT/MRI was assessed in vivo (e.g. chicken embryo). Au NPs@PEG-Mn2+ also demonstrated strong blood retention for at least 90 minutes following intravenous injection in mouse models. The promising performance of PEGylated PLAL-synthesized Au NPs containing manganese chelates could open new possibilities for the production of purer dual imaging contrast agents based on Au colloids.
|
| [49] |
DOI:10.1021/la503228v
URL
[Cite within: 1]
|
| [50] |
DOI:10.1039/c4tb00695j
URL
[Cite within: 1]
Dualmodal contrast agents of rare earth doped gadolinium oxide (Gd2O3) nanoparticles with high spatial resolution for magnetic resonance imaging (MRI) and high sensitivity for fluorescence imaging have attracted intensive attention in biomedical imaging. However, the rare earth doped nanoparticles mentioned above have been so far synthesized by the hydrothermal method, which is a bottom-up method, requiring high purity chemical reagents and relying on the availability of the respective precursors and strict reaction conditions. Here, we propose a facile and environmentally friendly top-down technique to synthesize the rare earth doped-Gd2O3nanocrystals at an ambient environment. Using this approach, we synthesize a series of Tm3+, Tb3+, and Eu3+doped-Gd2O3nanoparticle colloids and observe strong blue, green, and red visible fluorescence from the as-synthesized nanoparticle colloids. Cell confocal microscope images show that these synthesized nanoparticle colloids are good fluorescence imaging contrast agents. Taking Gd2O3:Eu3+nanoparticles as an example, we evaluate their performance in MRIin vitroandin vivo. These results indicate that the synthesized rare earth doped-Gd2O3nanocrystals can be used as MRI and fluorescence imaging dualmodal contrast agents. The developed technique is expected to be a general, facile and environmentally friendly strategy towards synthesizing rare earth doped nanoparticles for biomedical applications.
|
| [51] |
DOI:10.1021/jp301735c
URL
[Cite within: 1]
The development of nanoplasmonic sensing approaches for DNA detection based on the localized plasmonic properties of different metallic NPs fabricated by femtosecond laser ablation with an application for synthetic oligonucleotides as specific probes for genetic sequence variations is presented. The planar surface plasmon resonance (SPR) technique has been used to test oligonucleotide probes specific to rpoB genes of Mycobacterium tuberculosis. Optimal experimental conditions providing efficiency of hybridization between immobilized probe and cDNA target and performance of the SPR method were obtained and applied to the nanoplasmonic biosensing based on colloidal nanoparticles. Gold and silver/gold alloy nanoparticles were fabricated by the “pure” laser ablation method and have shown faster conjugation to thiol-modified DNA and higher stability in hybridization buffer than nanoparticles produced by chemical synthesis. Nanoparticle-enhanced and spectral SPR methods were used to confirm the efficiency of DN...
|
| [52] |
DOI:10.1021/acsnano.6b02627
PMID:27404114
URL
[Cite within: 1]
Therapeutically active small molecules represent promising nonimmunogenic alternatives to antibodies for specifically targeting disease-relevant receptors. However, a potential drawback compared to antibody–antigen interactions may be the lower affinity of small molecules toward receptors. Here, we overcome this low-affinity problem by coating the surface of nanoparticles (NPs) with multiple ligands. Specifically, we explored the use of gold and platinum nanoparticles to increase the binding affinity of Aβ-specific small molecules to inhibit Aβ peptide aggregation into fibrils in vitro. The interactions of bare NPs, free ligands, and NP-bound ligands with Aβ are comprehensively studied via physicochemical methods (spectroscopy, microscopy, immunologic tests) and cell assays. Reduction of thioflavin T fluorescence, as an indicator for β-sheet content, and inhibition of cellular Aβ excretion are even more effective with NP-bound ligands than with the free ligands. The results from this study may have implic...
|
| [53] |
DOI:10.1016/j.jpowsour.2015.11.078
URL
[Cite within: 1]
We have developed tandem laser ablation synthesis in solution-galvanic replacement reaction (LASiS-GRR) technique as a facile, green yet, efficient route to synthesize spherical PtCo NAs with varied sizes, compositions and degrees of alloying. The transformative concept here is the ability to design these nanostructured alloys by tuning the high-energy physico-chemical conditions emerging from liquid-confined, laser-induced plasma, and the solution-phase reaction pathways dictated by pH conditions and precursor salt concentrations. The resultant NAs exhibit uniformly alloyed cores with a Pt-rich shell of a few nanometers, as demonstrated by the electron energy loss spectroscopy (EELS) mapping. Such core鈥搒hell structure along with high degrees of alloying in these PtCo NAs promote their outstanding electrocatalytic ORR activities in acid electrolytes. Specifically, compared to commercial Pt/C catalysts, the PtCo NAs with Co molar ratio of 22.1% indicate a c.a. 3 and 6-fold increase in mass and specific activities respectively. Such enhanced ORR activities are attributed to the efficacy of tandem LASiS-GRR route to rationally tune size distributions/compositional ratios and alloying degrees of the NAs without the use of any surfactants or reducing agents that are otherwise indispensable in chemical synthesis methods, but harmful for catalytic performances.
|
| [54] |
DOI:10.1039/c3nr04715f
PMID:24217271
URL
[Cite within: 1]
To enhance the catalytic activity of gold nanoparticles (AuNPs) for the hydrogenation of nitro-aromatic chemicals, Pt was introduced into AuNPs to form "bare" PtAu alloy NPs using a physical approach, pulsed laser ablation in liquid (PLAL), on single metal-mixture targets. These PLAL-NPs are deemed to favor catalysis due to the absence of any surfactant molecules on their unique "bare and clean" surface. The PLAL-NPs were facilely assembled onto CeO2 nanotubes (NTs) by simply mixing them without conducting any surface functionalization, representing another advantage of these NPs. Their catalytic activity was assessed in 4-nitrophenol (4-NP) hydrogenation. The reaction catalyzed by alloy-NP/CeO2-NT catalysts demonstrates a remarkably higher reaction rate in comparison with that catalyzed by pure Au and Pt NPs, and other similar Au and Pt containing catalysts reported recently. A "volcano-like" catalytic activity dependence of the alloy NPs on their chemical composition suggests a strong synergistic effect between Au and Pt in the 4-NP reduction, far beyond the simple sum of their individual contributions. It leads to the significantly enhanced catalytic activity of Pt30Au70 and Pt50Au50 alloy NPs, outperforming not only each single constituent, but also their physical mixtures and most recently reported AuNP based nanocatalysts. The favorable d-band center shift of Pt after alloying, and co-operative actions between Pt clusters and nearby Au (or mixed PtAu) sites were proposed as possible mechanisms to explain such a strong synergistic effect on catalysis.
|
| [55] |
DOI:10.1016/j.apcatb.2012.11.030
URL
[Cite within: 1]
We report the preparation and catalytic properties of a new nanostructured catalyst, made of small (65502nm in diameter) and uniform gold nanoparticles (AuNPs) and ceria nanotubes (CeO 2 NTs). “Surfactant-free” AuNPs fabricated by pulsed laser ablation in liquid (PLAL) on a bulk Au target are efficiently assembled onto the surface of CeO 2 NTs without performing any surface functionalization of either component to promote their coupling, thanks to the presence of OH on the PLAL-AuNPs. The reduction reaction of 4-nitrophenol into 4-aminophenol catalyzed by our PLAL-AuNP/CeO 2 -NT catalyst exhibits remarkably higher reaction rate in comparison to that catalyzed by similar catalysts composed of chemically prepared AuNPs (Chem-AuNPs) as an active phase and/or commercially available CeO 2 powder as support. Their superior catalytic activity is found to be due to the unique, relatively “bare” surface of the PLAL-AuNPs as well as oxidized Au species induced by the strong interaction between the “barrier-free” surface of PLAL-AuNPs and surface defects (oxygen vacancies) of CeO 2 NTs. The important role of unique surface chemistry of PLAL-AuNPs in catalysis was further demonstrated in CO oxidation reaction in gas phase. Our results suggest that the use of PLAL-AuNPs enables easy and efficient attachment of AuNPs onto the surface of the CeO 2 NTs and their unique combination leads to the development of highly efficient catalysts. Our design and fabrication of the nanocatalysts take full advantage of the unique features of the PLAL-AuNPs and potentially constitute a general and efficient route to prepare other metal-NP/metal-oxide-support catalysts, which can therefore largely expand the applications of PLAL-noble metal NPs in catalysis.
|
| [56] |
DOI:10.1021/acsami.5b06153
PMID:26435201
URL
[Cite within: 3]
We report a simple and environmentally friendly route to prepare platinum/reduced graphene oxide (Pt/rGO) nanocomposites (NCs) with highly reactive MnOx colloids as reducing agents and sacrificial templates. The colloids are obtained by laser ablation of a metallic Mn target in graphene oxide (GO)-containing solution. Structural and morphological investigations of the as-prepared NCs revealed that ultrafine Pt nanoparticles (NPs) with an average size of 1.8 (卤0.6) nm are uniformly dispersed on the surfaces of rGO nanosheets. Compared with commercial Pt/C catalysts, Pt/rGO NCs with highly electrochemically active surface areas show remarkably improved catalytic activity and durability toward methanol oxidation. All of these superior characteristics can be attributed to the small particle size and uniform distribution of the Pt NPs, as well as the excellent electrical conductivity and stability of the rGO catalyst support. These findings suggest that Pt/rGO electrocatalysts are promising candidate materials for practical use in fuel cells.
|
| [57] |
DOI:10.1016/j.nanoen.2014.08.005
URL
[Cite within: 1]
We report a simultaneous strategy of impurity doping and crystal growth for building highly oriented Ge-doped α-Fe 2 O 3 nanosheet arrays vertically aligned on fluorine-doped tin oxide (FTO) glass substrates in hydrothermal environments, by using β-FeOOH nanorod arrays as sacrificial templates and highly reactive Ge colloidal solutions as dopant source. Microstructure characterization and elemental analysis reveal the preferential growth orientation, distribution of dopants, and doping level of Ge-doped α-Fe 2 O 3 nanosheet arrays. Based on the doping of Ge atoms, proper feature size of nanosheets (with thickness <1002nm) and preferential growth orientation within (001) basal plane of perpendicular nanosheet arrays, Ge-doped α-Fe 2 O 3 nanosheet arrays show a photocurrent density of 1.402mA02cm 612 at 1.2302V vs. RHE, which is more than 50 times of the undoped α-Fe 2 O 3 nanorod arrays. Moreover, we find that the annealing temperature remarkably affects the majority carrier density and optical absorption efficiency, which enabled the determination of photocurrent density of hematite nanostructure arrays.
|
| [58] |
|
| [59] |
DOI:10.1021/acscatal.6b02416
URL
[Cite within: 1]
Cobalt oxide is a cheap catalyst for oxygen evolution reaction; however, the low activity limits its practical application. Herein we report the preparation of highly active Co3O4 catalyst via a top-down process, laser fragmentation. The fierce laser irradiation generates fine and clean nanoparticles with abundant oxygen vacancies which simultaneously improve the adsorption energy and electrical conduction. Resultantly, the product achieves the best catalytic performance ever reported for cobalt oxide, even outperforming the noble-metal catalyst, RuO2.
|
| [60] |
|
| [61] |
DOI:10.1039/c5ra10854c
URL
[Cite within: 1]
This report concerns carbonaceous electrodes doped with tin(ii) oxide nanoparticles. Tin nanoparticles are obtained by pulsed laser ablation in water. Crystalline nanoparticles have been encapsulated in a carbonaceous matrix formed after pyrolysis of a mixture consisting of tin/tin(iv) oxide nanoparticles and gelatine. The obtained material is characterized by means of X-ray diffraction, selected area diffraction, scanning electron microscopy, transmission electron microscopy and energy dispersive X-ray analysis. Battery charging/discharging tests exhibit a capacity of 580 mA h g611for current densities of 100 mA g611. The cycling performance of the material suggests that the tested nanocomposite can be used as an anode for lithium-ion batteries.
|
| [62] |
DOI:10.1021/acscatal.5b01478
URL
[Cite within: 1]
We report a drastic enhancement of electrocatalytic activity toward glucose oxidation by using novel electrocatalysts on the basis of “bare” unprotected Au nanoparticles synthesized by methods of laser ablation in pure deionized water. The recorded current density of 2.65 A cm–2 mg–1 for glucose electrooxidation was higher than a relevant value for conventional chemically synthesized Au nanoparticles by an order of magnitude and outperformed all data reported in the literature for metal and metal alloy-based electrocatalysts. The enhanced electrocatalytic characteristics of laser-synthesized nanoparticles are explained by the absence of any organic contaminants or protective ligands on their surface, the relatively small size of nanoparticles, and their particular crystallographic structure. The employment of bare nanomaterials in glucose electrooxidation schemes promises a radical improvement in current biofuel cell technology and its successful application in bioimplantable devices.
|
| [63] |
DOI:10.1021/am3022976
PMID:23206317
URL
[Cite within: 1]
We report ligand-free synthesis of colloidal metallic nanoparticles using liquid-phase pulsed laser ablation, and electrophoretic deposition of the nanoparticles for fabrication of Cu(In,Ga)Se(2) (CIGS) thin film solar cells. First, colloidal metallic nanoparticles of Cu-In and Cu-Ga alloys are produced by pulsed laser ablation in common organic solvents without using stabilizing ligands. The nanoparticles are examined for phase, composition, and electrical surface charging and charge modulation mechanisms. Metallic precursor thin films with high purity and precise composition are produced by electrophoretic deposition of the colloids without transferring to another solvent and without using binders. Finally, we demonstrate fabrication of CIGS solar cells on Mo sheet substrates with an (active area) energy conversion efficiency up to 7.37%.
|
| [64] |
DOI:10.1002/elan.201400244
URL
[Cite within: 1]
AbstractA well-known limitation in the fabrication of metal-graphene composite has been the use of surfactants that strongly adsorb on the surface and reduce the performance of the catalyst. We demonstrate here a novel one-pot synthesis of gold nanoparticles by laser ablation of gold strip and in-situ decoration on graphene substrate. Not only the impregnation of nanoparticles was linker free, but also the synthesis by itself was surfactant-free. The composite materials were well characterized morphologically and functionally using electron microscopy, X-ray and electron diffraction, Raman spectroscopy, Zeta potential, electrochemical measurements and UV-Visible spectroscopic techniques. This linker-free gold-graphene based composite has been employed for catalytic applications pertaining to electrooxidation. We have explored the use of this composite as a binder-free electrode in electrocatalytic oxidation of methanol and ethanol in alkaline medium. Additionally, the onset potential for ethanol oxidation was found to be more negative, 6110061mV, an indication of its promising application in direct ethanol fuel cells.
|
| [65] |
DOI:10.1016/j.jpowsour.2014.08.023
URL
[Cite within: 1]
We report the synthesis of gold carbon nanotubes composite through a one-pot surfactant free approach and its utility for ethanol electrooxidation reaction (EOR). The method involves the application of laser ablation for nanoparticle synthesis and simultaneous assembly of these on carbon nanotubes. The catalyst has been characterized by field emission scanning electron microscopy (FESEM), energy dispersive X-ray analysis (EDAX) and UV–vis spectroscopic techniques. A systematic study of gold carbon nanotubes modified carbon paste electrode for EOR has been pursued. The kinetic study revealed the excellent stability of the modified electrode even after 200 cycles of EOR and with an Arrhenius energy as low as 6528kJmol 611 . Tafel slopes that are the measure of electrode activity have been monitored as a function of temperature of the electrolyte. The results indicate that despite an increase in the reaction rate with temperature, the electrode surface has not been significantly passivated by carbonaceous species produced at high temperatures.
|
| [66] |
DOI:10.1016/j.jpowsour.2013.09.045
URL
[Cite within: 1]
A series of mono dispersed Pt x Au 10061 x alloy nanoparticles (NPs), with x varying from 0 to 100, were prepared by pulsed laser ablation in liquids, using a series of targets that were made by mixing pure Pt and pure Au powders. The structures of Pt x Au 10061 x alloy NPs were assessed by transmission electron microscopy and X-ray diffraction. A face-centered solid solution is obtained over the whole composition range, and the particle size increases from 2.5 to 5.3 nm as x is increased from 0 to 100. The electrocatalytic performances of the Pt x Au 10061 x alloy NPs towards the formic acid oxidation were investigated by cyclic voltammetry and chronoamperometry. On as-prepared Pt x Au 10061 x alloy NPs, oxidation of formic acid occurs through dehydrogenation, while dehydration is the privileged mechanism on as-prepared mixtures of Pt and Au NPs. However, after a series of CV in 0.5 M H 2 SO 4 , both types of catalysts are able to oxidize formic acid according to the dehydrogenation pathway. After 600 s of electrolysis, the mass activities of Pt x Au 10061x alloy NPs is a factor of two larger than that of mixtures of pure Pt and pure Au NPs with the same surface composition, although both types of catalysts display similar activity with respect to the total electrochemically active surface area.
|
| [67] |
DOI:10.1016/j.snb.2012.11.059
URL
[Cite within: 1]
Laser ablated tin oxide quantum dots (SnO 2 QDs 651–502nm) uniformly dispersed in a colloidal solution have been electrophoretically deposited onto indium–tin-oxide (ITO) glass for immobilization of xanthine oxidase (XO x ) for fabrication of fish freshness biosensor. The results of electrochemical response studies conducted on XO x /SnO 2 QDs/ITO bio-electrode reveal higher sensitivity (0.514802μA/μM02cm 2 ), lower K m value (0.02202μM), faster response time (1002s), and wide linear range of 1–40002μM with regression co-efficient as 0.999, higher charge transfer rate constant 1.6302s 611 .
|
| [68] |
DOI:10.1166/jnn.2013.7123
PMID:23755572
URL
[Cite within: 1]
Abstract We report the application of nano crystalline tin oxide quantum dots (SnO2-QDs) for electrochemical detection of Vibrio cholerae based on DNA hybridization technique. SnO2-QDs (- 1-5 nm) have been synthesized by laser ablation technique in liquid (LAL) and electrophoretically deposited onto hydrolyzed surface of indium tin oxide (ITO) coated glass electrode. A single stranded oligonucleotide probe (23 bases) have been designed form the virulent gene sequence of V. cholerae and has been immobilized onto SnO2-QDs/ITO surface for the fabrication of ssDNA/SnO2-QDs/ITO bioelectrode and these bioelectrode have been further used for DNA hybridization (dsDNA/SnO2-QDs/ITO). The electrochemical response studies have been carried out with different concentration genomic DNA (100-500 ng/microL), which indicated that SnO2 provides an effective surface to bind with the phosphate group of DNA, thus resulting in an enhanced electron transport. The hybridized electrode exhibits linear response with regression coefficient (R) 0.974, high sensitivity 35.20 nA/ng/cm2, low detection limit (31.5 ng/microL), faster response time (3 s) and high stability of 0-120 days when stored under refrigerated conditions.
|
| [69] |
DOI:10.1088/0957-4484/27/21/215501
PMID:27079452
URL
[Cite within: 1]
Abstract We report the fabrication and characterization of a surface plasmon resonance (SPR)-based fiber optic sensor that uses coatings of silver and aluminum (Al)-zinc oxide (ZnO) core-shell nanostructure (Al@ZnO) for the detection of phenyl hydrazine (Ph-Hyd). To optimize the volume fraction (f) of Al in ZnO and the thickness of the core-shell nanostructure layer (d), the electric field intensity along the normal to the multilayer system is simulated using the two-dimensional multilayer matrix method. The Al@ZnO core-shell nanostructure is prepared using the laser ablation technique. Various probes are fabricated with different values of f and an optimized thickness of core-shell nanostructure for the characterization of the Ph-Hyd sensor. The performance of the Ph-Hyd sensor is evaluated in terms of sensitivity. It is found that the Ag/Al@ZnO nanostructure core-shell-coated SPR probe with f0002=00020.25 and d0002=00020.040 0204m possesses the maximum sensitivity towards Ph-Hyd. These results are in agreement with the simulated ones obtained using electric field intensity. In addition, the performance of the proposed probe is compared with that of probes coated with (i) Al@ZnO nanocomposite, (ii) Al nanoparticles and (iii) ZnO nanoparticles. It is found that the probe coated with an Al@ZnO core-shell nanostructure shows the largest resonance wavelength shift. The detailed mechanism of the sensing (involving chemical reactions) is presented. The sensor also manifests optimum performance at pH 7.
|
| [70] |
DOI:10.1021/la1033146
PMID:20942423
URL
[Cite within: 1]
In this work, diverse hollow nanoparticles of metal oxides and sulfides were prepared by simply laser ablating metal targets in properly chosen liquids. The Kirkendall voiding and the selective heating with an infrared laser were shown to work as two independent mechanisms for the formation of such hollow nanoparticles in only one- or two-step synthesis approaches. One of the prepared materials, ZnS hollow nanoparticles, showed high performance in gas sensing. The simple, fast, inexpensive technique that is proposed demonstrates very promising perspectives.
|
| [71] |
DOI:10.1002/admi.201500801
URL
[Cite within: 1]
Here an alternative preparation route, pulsed laser ablation in liquid (LAL) of FeCl3 solution, is reported to facilely synthesize crystalline iron oxychloride (FeOCl) nanosheets at ambient conditions. Well-dispersed spherical gold (Au) nanoparticles (NPs) are simultaneously decorated on surface of the FeOCl nanosheets, which possess (010) preferred orientations with microsized dimensions in planar and tens of nanometers in thickness. The crystalline size and composition of the Au/FeOCl can be effectively modulated by simply changing FeCl3 concentrations. Technical observations illustrate that the nanocomposites possess good thermal stability and surface of which adsorbs abundant H2O molecularly and oxygen species chemically. The FeOCl nanosheets are formed through chemical side hydrolysis reaction in the localized liquid region with gradient temperature, which is derived from thermal transfer of LAL-induced plasma plume. The Au/FeOCl nanocomposites, as chemiresistors, show exceptionally high sensing response and perfect selectivity to HCl gas at room temperature. The excellent sensing behavior is ascribed to the Au-NP-enhanced surface chemisorption of oxygen species and selective adsorption of HCl for FeOCl nanosheets. The synthesized Au/FeOCl nanocomposites provide a new candidate for exclusive HCl detection. And the proposed LAL-assisted fabrication routes might open new perspectives for formation of other metal oxychloride compounds.
|
| [72] |
DOI:10.1039/c2nr32078a
PMID:23069859
URL
[Cite within: 1]
Abstract Porous tungsten oxide (WO(3)) nanoflakes have been synthesized by a simple and green approach in an ambient environment. As a precursor solution a polycrystalline hydrated tungstite (H(2)WO(4)00·H(2)O) nanoparticles colloid was first prepared by pulsed-laser ablation of a tungsten target in water. The H(2)WO(4)00·H(2)O nanoflakes were produced by 72 h aging treatment at room temperature. Finally, porous WO(3) nanoflakes were synthesized by annealing at 800 00°C for 4 h. Considering the large surface-to-volume ratio of porous nanoflakes, a porous WO(3) nanoflake gas sensor was fabricated, which exhibits an excellent sensor response performance to alcohol concentrations in the range of 20 to 600 ppm under low working temperature. This high response was attributed to the highly crystalline and porous flake-like morphology, which leads to effective adsorption and desorption, and provides more active sites for the gas molecules' reaction. These findings showed that the porous tungsten oxide nanoflake has great potential in gas-sensing performance.
|
| [73] |
DOI:10.1039/c3ce40787j
URL
[Cite within: 1]
Ag2V4O11 brush-like nanostructures with a large specific surface area have been synthesized by a unique electrochemistry assisted laser ablation in liquids without any template or surfactant in an ambient environment. Considering the large surface-to-volume ratio of the as-synthesized nanostructures, an Ag2V4O11 brushlike nanostructured gas sensor was fabricated and exhibited an excellent performance in sensor response to ethanol concentrations in the range of 10 to 600 ppm under low working temperature. This high response was attributed to the highly crystalline and brush-like surface, which leads to the effective adsorption and desorption, and provides more active sites for gas molecules reacting. These findings show our desire to encourage the synthesis of new nanomaterials, e. g. ternary transition metal oxides, as gas sensors prepared by novel means.
|
| [74] |
DOI:10.1039/b918759f
PMID:20379485
URL
[Cite within: 1]
Although nanomaterials investigations have been carried over the recent decades, researchers still face a fundamental challenge: how to control the phase, size and shape of nanocrystals in the synthesis of nanomaterials, i.e., how to achieve the transformation from nanocrytsal synthesis to functional nanostructure fabrication. For this issue, we, in this review, introduce recent developments in laser ablation in liquid (LAL) for the synthesis and fabrication of novel nanostructures with metastable phases and shapes. Laser ablation of solid targets in liquid has actually opened a door toward to synthesize nanocrystals and fabricate nanostructures due to these advantages as follows: (i) LAL is a chemically "simple and clean" synthesis due to the process with reduced byproduct formation, simpler starting materials, no need for catalyst, etc. (ii) Under ambient conditions, not extreme temperature and pressure, a variety of metastable phases that may not usually be attainable, can be generated by mild preparation methods. (iii) New phase formation involves in both liquid and solid upon LAL, which allows researchers to choose and combine interesting solid target and liquid to synthesize nanocrystals and fabricate nanostructures of new compounds for purpose of fundamental research and potential applications. (iv) The phase, size and shape of the synthesized nanocrystals can be readily controlled by tuning laser parameters and applying assistances such as inorganic salts or electrical field upon LAL. For example, we have synthesized the micro- and nanocubes of carbon with C(8)-like structures by the inorganic salts assisted LAL, and the micro- and nanocubes and spindles of GeO(2) by the electrical field assisted LAL. Additionally, we have developed a new technique to fabricate functional nanopatterns on the basis of the pulsed-laser deposition in liquid. Accordingly, LAL could greatly extend its application in fabrication of functional nanostructures in the future.
|
| [75] |
DOI:10.1016/S0009-2614(02)00871-0
URL
[Cite within: 1]
Diamond nanocrystals with cubic and hexagonal structures have been prepared by pulsed laser induced liquid–solid (acetone1–graphite) interfacial reaction (PLIIR) at normal temperature and pressure. The structure and morphology of the resulted diamond nanocrystals were characterized by transmission electron microscopy (TEM). The Raman spectroscopy analysis of the obtained diamond nanocrystals showed that three diamond Raman lines, i.e., 1307, 1100, and 623 cm 611 were observed. Interestingly, the Raman line at 1100 cm 611 was identified to be original from nano-diamond. In addition, a new Raman line at 926 cm 611 due to diamond was observed in the Raman spectra (RS).
|
| [76] |
DOI:10.1016/j.cplett.2003.10.004
URL
[Cite within: 1]
Nanorods of the immiscible alloying silver–nickel were synthesized by pulsed-laser induced liquid/solid interfacial reaction (PLIIR). Typical diameters of these nanorods were in the range of 30–50 nm, and lengths were in the range of 300–500 nm. Transmission electronic microscope (TEM) equipped with energy depressive X-ray spectrometer (EDS) and selected area diffraction (SAD) were employed to characterize these nanorods. The analysis results showed that the prepared Ag–Ni alloying nanorods were single crystals with fcc structure. Eventually, we proposed the growth mechanism of the immiscible alloying nanorods upon PLIIR, in which both liquid and solid were simultaneously involved.
|
| [77] |
DOI:10.1557/JMR.2003.0387
URL
[Cite within: 1]
Boron nitride (BN) nanocrystals with explosion (E) phase were prepared by a novel laser-assisted materials fabrication, i.e., pulsed-laser-induced liquid (acetone)/solid (hexagonal boron nitride bulk) interfacial reaction at normal temperature and pressure. Typical diameters of these synthesized quasi-spherical BN nanocrystals were in the range of 30 to 80 nm. Transmission electron microscopy, x-ray diffraction, and Fourier transformed infrared spectroscopy were used to identify the morphologies and structures of the synthesized nanocrystals. Additionally, we proposed the formation mechanism of cubic-BN and E-BN nanocrystals upon pulsed-laser-induced liquid/solid interfacial reaction, in which both liquid and solid were simultaneously involved.
|