本章对人工纳米材料(engineered nanomaterials,ENMs)废弃物在焚烧过程中的表现及暴露情况的研究结果进行了概述,同时也对含纳米材料废弃物(waste containing nanomaterials,WCNMs)在处理方面存在的知识空白进行了总结。本报告介绍了废弃物焚烧过程中ENMs行为表现的科学信息,概述了城市生活垃圾焚烧炉(municipal solid waste incinerators, MSWI)中ENMs的高度相关性,简要描述了废物焚烧的最优实用技术(best available techniques,BAT)及阻滞或销毁危害物质的技术,并探讨了ENMs穿越当前污染控制装置的可能途径。
本章对废弃物焚烧过程中人工纳米材料的表现及暴露情况的研究结果进行了概述,并对WCNMs处理方面存在的知识空白进行了梳理。
本章不仅简要解释了纳米技术的相关性,而且呈现了有关人工纳米材料数量和主要来源的相关信息。此外,本章还揭示了废弃物焚烧的过程与可用标准,同时调查研究了人工纳米材料的去向和行为表现。本章对现有的研究结果进行了总结,并对未来的研究工作做了展望。
本报告认为,想要估计废弃物中ENMs的数量,关键在于获取市场上含有ENM的产品的有效信息。此外,对于废弃物焚烧过程中ENMs产生的影响和行为依然所知甚少;不仅如此,许多与WCNMs焚烧相关的文献和研究成果是相互矛盾的。因而,深入的科学研究显得尤为必要。当前阶段,为了避免人工纳米材料对人类以及周围环境的危害,正如欧盟BREF文件中所提及的,所有的废弃物焚烧工厂都应该安装废气处理系统。此外,废弃物焚烧后留下的固体残留物的处理也需要进一步调查研究。
纳米技术是一个相对年轻且充满希望的应用领域。ENMs在许多领域得到了广泛应用,例如制药、化妆品、电池、油漆、涂料或作为某些材料和产品的添加剂用以提高材料或产品的某一特性。纳米技术给我们日常生活带来的益处不容小觑。但是,目前科学研究表明,一些ENMs在社会生活中的应用对人类健康和环境是有害的,只是更加具体的相关信息却并不明确,因此,逐个案例的暴露评估仍然是目前推荐的研究方法[95]。
随着一些含有ENMs的产品的报废,纳米材料可能随之进入到周围的环境中。由于纳米技术是新兴的科学领域,人们对含人工纳米材料废弃物的处理还没有给予足够的重视。因此,我们依然不清楚未来能否实现WCNMs的环境无害处理。鉴于此,专家们担心某些产品可能会将人工纳米材料排放到不同的环境当中。
本章的内容仅着眼于一种废弃物处理方式——焚烧,相关的讨论主题都以此为基础展开,包括:
◆ 废弃物焚烧过程中ENMs行为表现的主要科学信息;
◆ 生活垃圾焚烧炉(MSWI)中ENMs的高度相关性的概述;
◆ 阻滞或销毁危害物质的最优实用技术(BAT)之简述;
◆ ENMs穿越当前污染控制装置的可能途径之简论。
本部分为讨论废弃物焚烧炉中的ENMs提供了一些必要的背景知识,包括ENMs的数量、来源以及进入到生活垃圾焚烧炉中的纳米材料的数量。
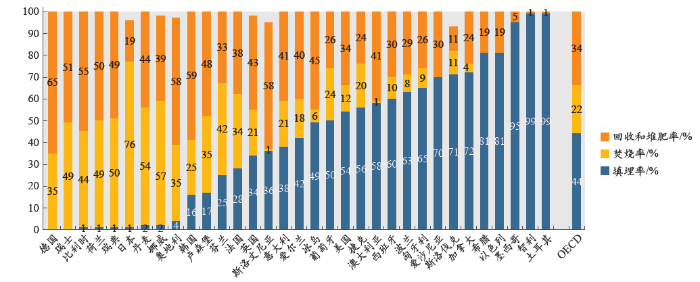
过去几年间,世界各地的废弃物焚烧工厂的数量呈上升态势。据OECD 2014年统计[96],在2012年,OECD国家共有大约6.58亿吨的生活垃圾废弃物,其中的22%大约1.45亿吨被焚烧。更加详细的数据请参阅图5.1。
注:数据是2009至2013年每个国家的最新数据。新西兰没有可用数据。 由于不包括其他回收和处置处理,所以所列类别的总和可能不到100%。
来源: OECD (2016), “Municipal waste”, OECD Environment Statistics (database), http://dx.doi.org/10.1787/data-00601-en (Accessed on 19 January 2016).
正如上文所述,含ENMs的产品数量在稳步增长。一些研究表明,2010年TiO2、ZnO、SiO2、FeOx、AlOx成为ENMs市场上的主流产品,主要应用在涂料、油漆、颜料、电子及光学器件、化妆品、能源及环境应用如催化剂[97]。表5.1列出了全球、欧洲、美国与瑞士的一些ENMs的产量。当ENMs产品到达使用期限后,它们将统统成为废弃物。例如,Musee发现[98],2006至2010年间TiO2产量为5 000吨/年,但在2011至2014年间几乎达到1万吨/年。大量但具体数量不详的ENMs将会进入废弃物焚烧工厂。
进入生活垃圾焚烧炉中的人工纳米材料有两个主要源头。第一个是生活垃圾(包括一些含人工纳米材料产品的制造过程中所产生的残余物)。第二个是能进行焚烧的污泥。
迄今为止,关于含有人工纳米材料的产品或其数量的信息颇为有限。然而含有ENMs的产品(例如食品包装、清洁产品)在报废后不得不进行处理,其势必会进入生活垃圾焚烧炉中。对于人工纳米材料生产中所产生的残余物采用同样的处理方式[102]。
据Musee估计[98],化妆品中所含纳米材料的95%最终进入了废水。Kuhlbusch与Nickel证实了在清洗含有人工纳米材料衣物或纺织品的时候有纳米银的排放[103]。如果废水由废水处理厂处理,ENMs大部分会转移到废水污泥中,因此,仍然存在于废物处理流程中。Burkhardt等人指出有93%~99%的纳米银转移到了废水处理后的污泥中[104]。
如果污泥被当作肥料使用,则ENMs进入土壤是非常有可能的。如果对污泥进行回收,那么ENMs就会进入焚烧工厂。
Roes等人[105]计算了每吨城市生活垃圾焚烧后排放气体中所含的ENMs量,假设纳米组分中的ENMs含量在1%与10%(重量百分比)之间。假定生活垃圾中的平均塑料含量为12%,其中7%为纳米组分。然而,这需要考虑投送到MSWI中的废弃物的不同材料的平均含量差异巨大,原因在于不同地域的基础设施以及进入到各自的MSWI工厂的具体废物处理流程都大不一样。
大多数生活垃圾焚烧工厂都建有废弃物存储槽,通常情况下是水泥床。存储槽中的废弃物将被充分搅拌以便能够有效燃烧。最常见的焚烧技术就是层燃系统。最后会产生又热又含有大量高污染组分的烟气,烟气流将通过一个蒸汽发电机用以发电。烟气随后进入到烟气净化系统,在这里尘、酸以及其他有害物质将通过化学过程得以消除,或通过物理过程予以分离。清洁后的烟气最终通过一个高高的烟囱进入到大气中,所以极有可能会有部分ENMs进入到大气中。焚烧后的残余物,称作底灰,可以被应用于道路建设,或者被填埋。从烟气净化系统中产生的飞灰也含有ENMs,通常的处理方法就是填埋。
废弃物焚烧工厂中BAT的采用是一个比较复杂的话题,主要是由于世界各地的工厂设施以及当地环境和气候条件存在着差异。为了解决这一问题,欧盟建立了一个各行业涉及废弃物焚烧的BAT信息交流机制。信息交流的成果记录在最佳实用技术参考文献中(best available technique reference document,BREF)。
文献BREF指出,为了保护人类健康和环境安全,所有的废弃物焚烧工厂都应安装废气处理系统;而且,为了满足几个欧盟法令的要求,所有欧盟的废弃物焚烧工厂都已安装废气净化系统[比如2010年11月4日的欧洲议会和理事会关于工业排放的2010/75/EU法令(综合污染防控,integrated pollution prevention and control,IPPC),OJL334,17.12.2010, p.17]。法令规定了一些气态污染物的排放限值,比如尘、氮氧化物(NOx)、二氧化硫(SO2)、重金属、二氧化物、呋喃及其他。法令还规定了对烟道气净化后产生的废水如何排放的相应要求。然而,BREF中有关废弃物焚烧的内容并没有提及ENMs。因此,BREF的内容只能作为高标准废气治理的参考,而为了有效处理ENMs,应该对BREF文件中关于标准的描述加以修订及完善。接下来,我们有理由相信一旦获取了生活垃圾焚烧炉废气中ENMs去除的可靠数据,用于ENMs去除的最优实用技术就会出现在BREF文件中。
迄今为止,对于MSWI中ENMs排放的研究甚少。但根据这些极少的研究成果可知,高水平的末端废气治理系统能够有效去除大部分的ENMs。然而,这仅表现在某些特定材料上,或者是基于模型的计算。
因此,德国联邦环境机构/德国联邦环境部与德国联邦研究部已经开展了焚烧过程中纳米粒子排放的科学研究。遗憾的是,目前为止还未见相关研究成果的报道。
然而,依然有许多国家对于废气的治理并没有执行BAT标准技术。烟道气治理不仅特指ENMs,而是包括所有排放物能够得到有效控制,各国政府部门应当要求所有废弃物焚烧工厂都实施严格的处理标准。
生活垃圾焚烧炉中ENMs的最终归宿极大地受反应条件、废弃物槽以及燃烧室温度的影响[105]。这些因素都会影响ENMs从MSWI中的排放。
焚烧过程中,ENMs的重构或破坏途径大概有5种可能。
(1)ENMs由于燃烧被破坏(例如CNT变成CO2)[106]。
(2)ENMs没有被破坏或焚烧掉,而是被废气治理系统捕捉到(例如金属氧化物)。事后可以在飞灰或其他残留物中检测到这些ENMs。
(3)某类型的ENMs并没有在燃烧过程中被破坏掉,然而,它们却与别的物质反应形成了新的粒子(例如CaCO3生成CaO 与 CO2或 ZnO + HCl生成 ZnCl2+ H2O)。
(4)较大的颗粒分解变成了新的更小的粒子或者甚至是ENMs。Roes等人[105]研究报道了ENMs如何在焚烧过程中被破坏或转变成其他ENMs,亦或没有任何改变。
(5)ENMs聚集成更大的粒子,从而失去了纳米的状态。
本报告重点关注前两种情况。为了填补有关废弃物焚烧过程中ENMs终极结局的科学空白,开展更多的科学研究是有必要的。德国联邦环境机构开展了一项有关废弃物焚烧后以及污泥焚烧工厂中纳米尺度TiO2最终结局的科学研究。此项研究利用质量平衡进行分析,在2015年公布了相应的研究成果。法国同样也开展了两项研究工程,旨在评估WCNMs燃烧的排放物以及废气治理系统的效率。研究结果指日可待。
2012年Walser等人发布了一项针对实际废弃物焚烧工厂中ENMs焚烧的调查报告,分析了氧化铈(平均80 nm)在焚烧过程中的具体情况[107]。
实验设计了两种情况:第一,ENMs被喷淋在废弃物上;第二,ENMs被直接置入燃烧炉中。第一种情况的质量平衡表明,几乎81%的ENMs转成了炉渣,几乎19%变成了飞灰,0.02%进入到冷却水中,仅有0.0004%进入到干净的废气中。第二种情况,53%的ENMs在炉渣中,45%在飞灰中,1.7%进入到冷却水中。
Walser等人[107]指出静电沉降与湿法废气净化系统一起使用可以有效去除纳米氧化物。因此,如果废弃物焚烧工厂安装了此类的废气治理系统,纳米CeO2将会得到极为有效的控制。
与此相反,Roes等人[105]则认为去除效率仅仅针对大于100纳米的粒子非常高,但对于小于100 nm的粒子,去除效率会出现大幅下滑的现象。小于100 nm的粒子可以部分被纤维过滤器和湿式除尘器捕捉,然而,依然会有高达20%的粒子逃脱。被除尘器捕捉到的ENMs将被保留在残余物中(底灰与飞灰)。Roes等人[105]认为残余物不需要进一步过滤处理。然而,应当引起注意的是去除效率将会因过滤技术、过滤材料的不同而不同,因此每个工厂的数据都可能不一样。而且,这个说法并不是通过实验数据所得,只是理论研究而已。
Mueller等人[106]发现,大部分ENMs都会在焚烧后进入底灰并且最终进入填埋场。其他的残余物比如飞灰,数量规模要远小于底灰。
Mueller等人认为废弃物焚烧对ENMs有非常重要的影响。大多数ENMs会在焚烧后进入残余物且被掩埋,但是碳纳米管的表现却不一样。由于独特的化学性质,大约94%的碳纳米管将会被烧掉。然而,一些现有数据表明碳纳米管被烧掉的效果并没有如期望的那般好[108]。
Mueller等人[106]估算在瑞士的废弃物与污泥焚烧工厂大约会产生总量为8万吨/年的飞灰,其中仅有0.00058%重量比的飞灰是小于100 nm的纳米材料,即464千克/年。与此不同的是,Mueller等人[109]的模型计算结果却足有50倍之高:22吨/年TiO2,0.8吨/年ZnO,160吨/年Ag与4.9吨/年CNT。同样,Buh等人[110]调查了5家装有静电沉降器或袋式过滤器的废弃物焚烧工厂,0.00009%~0.07%的飞灰为纳米材料。他们也认为烟道气中的纳米材料占比明显比模型结果要低很多。造成测量与模型结果差异的原因可能是ENMs容易聚集且会快速形成更大的几百纳米的粒子。因此,根据欧盟的规定,它们不再是ENMs。
欧盟指出含有碳纳米管的纺织品的燃烧会排放ENMs,只有当焚烧温度超过850℃时才能破坏掉这些碳纳米管。因此,为了达到如此高的焚烧温度,需要建立现代化的、运行良好的废弃物焚烧工厂[111]。
荷兰卫生委员会认为[102],虽然MSWI会排放超细粒子,但这与交通产生的排放物相比简直不值一提。超细粒子浓度在经过烟道气除尘装置后会降低为原来的千分之一,但是仍然会有较高数量的小粒子无法被捕捉从而进入到大气中。依据当前这些有限的证据,委员会认为焚烧过程会排放出ENMs的说法目前看来貌似有些道理。
未来含有ENMs的产品数量很可能会增加,因此含有ENMs的废弃物数量也会增加。关于产品中ENMs影响的信息在当前看来实在太少。为了能够估量废弃物中ENMs的规模,获取市场上含ENMs产品的信息就显得至关重要。
不仅如此,人们对在焚烧过程中ENMs到底处于什么样的状态也知之甚少。而且当前许多有关WCNMs的科研报道也存在相互不一致的地方。一方面,有研究报道说对废弃物焚烧炉的测量没有发现ENM的排放;另一方面,模型计算得出的结论认为ENMs能够穿越净化装置。因此,需要进一步的科学研究。令人欣慰的是相应的科学研究已经展开。
然而,为了保护人类健康以及环境,所有的废弃物焚烧工厂都应当安装废气治理系统,正如BREF所描述的一样。从目前的研究可知,如果工厂能够安装BAT废气治理系统,绝大部分的ENMs都会被这套系统所捕获。然而,这种表现仅限于纳米CeO2;其他推测全部基于理论考虑。此外,所有的焚烧参数,比如温度、滞留时间或氧浓度都应该加以充分考虑,以求能够达到破坏或去除ENMs的最佳效果。
不幸的是,当今世界依然有许多国家的废弃物焚烧工厂没有安装完善的废气处理系统,不仅对于ENMs如此,对于其他排放物也是如此。因此,各国政府有责任确保在MSWI工厂中执行高标准的废气治理系统。
对于生活垃圾焚烧中ENMs的表现以及它们是如何排放到周围环境中的,目前的理解还处于初级阶段。为了获取更多的相关信息、知识或数据,对不同的ENMs在不同的废弃物焚烧工厂的更加详细的研究调查是非常有必要的。这些研究应该包括对有效去除ENMs设置参数或条件的摸索确认。而且,焚烧后固体残余物中ENMs的结局也需要进一步研究,不容忽视。
2.“a”瑞士括号中的数值是通过利用瑞士(690万)与欧洲(5.93亿)的人口数值推算所得。
来源:参考文献[99]、[100]、[101]。
本章根据现有科学信息对填埋处理方式下ENMs的来源、结局、表现以及填埋处理效率进行了回顾。并调查研究了ENM排放到环境的潜在途径以及由此带来的相关风险。本章还总结了关注重点,且识别出当前存在的知识空白。
纳米技术工业的快速发展给人类社会提供了大量的含ENMs的产品,便利人民的生活的同时也给废弃物的处理带来了新的挑战。当前阶段,关于纳米材料废弃物处理的相关知识非常有限,尤其关于这些废弃物对人类健康和环境的危害知之甚少。快速发展的工业增加了产品的数量和多样性,同时这些产品报废后在后续的废弃物处理过程中增加了ENMs的规模。多项研究表明,有相当分量的ENMs将通过填埋处理,因此专业人士建议,应该对与此相关的废物流、环境风险、目前废弃物管理的实践和技术的有效性给予更多的关注,以期提高对此领域的科学认识,最终目的则是为了阻止纳米材料对人类和环境潜在的危害[112,123]。
本报告旨在对垃圾填埋中ENMs的来源、结局、呈现情况以及处理技术的效果进行分析整理,并最终给出有用的科学信息。由于ENMs进入环境的途径有多种,因此研究中有许多复杂的因素需要考虑。这是一个正在开展中的新兴领域,而且有关纳米毒理学的研究存在着较大争议,包括对ENMs特征的定义,ENMs化学成分以及形状、尺寸、结构的不同所产生的潜在的毒性差异。此外,一旦排放,有一大部分ENMs可能会改变,这也是需要考虑的因素[116]。虽然并不是所有的ENMs都有危害,但科研人员一致认为从预防的角度讨论ENMs填埋处理方式所带来的危险是有必要的。在呈现这些研究成果的同时,本报告也对其警示性看法做出了归纳,但这些内容可能是正确的,也可能是错误的,未来会有更多的科学发现不断冲击现有的科研成果。最后,为了对ENMs的填埋提供科学决策,本报告通过关键点的总结以及知识空白的识别,试图为相关讨论搭建一个科学的平台。
陆上废弃物堆积与填埋是全球范围内应用最为广泛的废弃物处理技术。但是处理的标准和实际操作情况却大不相同,既有不受控的情况,也有高度专业化工程化的填埋。填埋气与渗出液中污染物的潜在排放很大程度上依赖于填埋场的设计、条件以及控制措施的复杂程度,包括填埋气与渗出液的收集与处理系统。
现代化的填埋场很少依赖天然防护,而是采用综合性的防护措施,确认防护边界并配备渗出液与填埋气的收集系统。收集系统的目的在于捕捉与处理渗出液和填埋气,从而阻止渗出液转移到地表水,或者阻止没有经过处理的填埋气排放到大气中。没有经过现代化技术装配的填埋场因缺乏对环境的控制,存在潜在污染物环境暴露风险,因而被认为是不受控的废弃物处理场所。
由于ENMs在大量产品中的广泛使用,部分ENMs有可能通过填埋气排放;但是,本报告的关注点将放在填埋渗出液中的ENMs,因为其被认为是ENMs脱离填埋场的主要途径。然而,对填埋气中ENMs特征的研究仍将是未来有待开展的一项重要工作。
当雨水冲刷堆积如山的废弃物时,或者填埋场中的废弃物自行分解流出液体的时候,都会产生渗出液。渗出液的组成成分极其复杂,这依赖于被填埋的废弃物的类型、沉积物的数量、填埋场的构造与管理、填埋场的使用寿命以及其他因素,例如pH、温度与微生物等。渗出液化学成分的变化同时也受家庭垃圾和其他废弃物中产品的化学物质多样性的影响。此处所说的其他废弃物来自轻工业、商业与研究活动,包括构造、修复与破坏建筑形成的垃圾、被污染土壤、灰与废水污泥,其中均有可能含有ENMs(详见 4.2.1)。
填埋在国际科学研究领域一直保持着较高的受关注度[124-126],这是因为人们担心填埋场里的污染物如果排放会对环境带来极为不利的影响。加拿大有一项执行了多年(2008–2013)的研究项目,在选定的生活垃圾填埋场里提取一些渗出液进行检测,发现了一些主要的宏观尺度化学物质1(1 宏观尺度化学物质是指传统化学领域中肉眼可以观察到的物质。与此相对应的是微观或纳观化学物质,其尺寸在几微米或几纳米,肉眼无法观察到,只能借用放大仪器如光学显微镜或更强的放大设备如电子透镜或扫描隧道显微镜辅助才能观测到。微观大约在单个活细胞的尺度上,而纳观大约在单个原子或分子的尺度上。www.cengage.com/resource_uploads/downloads/1439049300_222029.pdf, http://chem.sci.utsunomiya-u.ac.jp/v10n2/MashitaA/MashitaA_body.html.)。研究结果表明,传统的现场处理工艺以及废水处理系统不能有效地处理各种条件下产生的渗出液中的一些物质[127,128]。这项研究虽然并不包括ENMs,但却说明了处理效率的不稳定性。Hennebert等人[129]最近的一项研究表明在各种废弃物产生的渗出液中出现的ENMs,大多是以胶体形式出现(分散相的尺寸范围为1nm~1μm),且与自然胶体的元素成分不同。
虽然在渗出液中检测到的很多物质浓度并不高,但目前对这些物质可能带来的污染综合性效应却知之甚少,也没有对所有可能的物质进行详尽分析。填埋过程中对ENMs的处理会给废弃物管理系统带来前所未有的复杂性、不确定性以及危险性,这是因为管理系统并不是针对所有污染物而设计的[127]。虽然在某些情况下,传统的现场渗出液处理系统在处理一些物质时表现出了有效性,但对特定的化学物质或者不同条件下ENMs的去除却没有效果。因此,研究填埋中ENMs的风险、排放方式以及后续可能会对环境和人类健康带来的影响都十分必要。本报告中的相关内容力求能为未来废弃物处理的管理决策以及解决方案提供有益的信息。
生活日用、工业与医疗行业的产品革新为ENMs的使用提供了机会,它们的身影出现在了化妆品与个人护理产品、衣物与纺织品、抗菌药物、抛光清洗与黏合剂、太阳电池、汽车和航空用轻量高强度塑料、防腐剂、食品处理与食品包装中[130-132]。据2014年的《新兴纳米技术工程》报告,截止到2013年10月份,纳米产有1 628种品,其中健康产品比重最大,占比48%。健康产品又以化妆品和个人护理产品为主,占比37%。有关确定填埋场中以哪种纳米产品(或ENMs)为主、表征相应风险或量化ENMs等不在本报告的研究范围内。然而,已经有些研究人员开始推动此方面的研究工作。这些后续研究,包括ENMs的分类以及危害识别,必将会为今后更加深入的研究提供指导和帮助。
英国标准研究院(British Standards Institution, BSI)的英国标准指南PD 6699-2区别了4种类型的纳米材料废弃物(固体和液体):
纯纳米材料;
被纳米材料污染的物件,如容器、抹布、一次性个人防护装备;
含纳米材料的悬浮液;
带有纳米材料的易碎固体物或表面附着有纳米结构的固体物,在与空气或水接触时,或者在遇到外力的情况下,纳米材料很容易与原固体基体分离[133]。
生活垃圾填埋中ENMs的重要来源是使用报废后的消费类产品[112,114,116,122,134,135]。一份利用生命周期分析方法的研究估算,有3种重量百分比超过50%的ENMs(纳米银、纳米二氧化钛和碳纳米管)最后会进入填埋场[120]。Keller等人[116]估计,2010年全球ENMs产量26~30.9万吨,其中绝大部分(63%~91%)可能通过填埋场处理。从ENMs来源的重量比考虑,最大的纳米产品来源是塑料制品以及建筑材料[116,136]。
有害废弃物填埋场以及生活垃圾填埋场中工业纳米废弃物的处理不应当被忽略。例如,根据环境污染皇家委员会(2008)的信息,在富勒烯的生产过程中仅有10%的原料被使用,其余的则进入到填埋场处理。Boldrin等人[114]提供的数据也同样表明,在几个案例中,制造过程中产生的废弃物的量要远远大于最终的ENMs产品量。然而,这并不代表其他ENMs生产过程的特征,因为资源浪费显著,这更可能是一个糟糕的经济成本控制案例。虽然没有结论性的研究成果,但表明了来源于ENMs制造过程的纳米废弃物应当被优先考虑[114]。
除了上述ENMs来源,焚烧炉以及废水处理工厂也会通过灰、渣或生物固体将ENMs转移到填埋场。在污泥稳定或焚烧期间保留和/或转化的纳米粒子能进入填埋场渗出液中[137,138]。虽然在焚烧过程中避免纳米粒子进入大气是可能的,但是据观察含有纳米粒子的残余物最终会出现在填埋场[139]。Mueller等人[138]对瑞士的废弃物中ENMs的运动轨迹进行了分析,他们认为主要的ENM运动途径是以废弃物焚烧工厂底灰的形式进入到填埋场。生物固体也是填埋场中ENMs的重要来源。据估计,进入废水处理厂的纳米二氧化钛大约有四分之三最终会出现在填埋场。同样,每年平均有4.77吨纳米银出现在填埋场[120]。另一个需要考虑的纳米废弃物来源是污水排放或废气排放中的ENMs。因治理而产生的其他形式的纳米废弃物也需要正确处置[140]。
总之,大量的各种各样含有ENMs的消费类纳米产品、制造业中的纳米废弃物以及其他废弃物管理系统产生的残余废弃物产品都会在填埋场中进行处理。作为许多ENMs可能最终的归属地[116,141],填埋需要得到特别关注。作为处理ENMs的最后手段或者作为ENMs暴露至环境的一条途径,填埋到底做到什么程度才算成功需要更加深入的研究。
由于ENMs内在的化学构成、形状、尺寸与结构,它们会展现出比较清晰的自我痕迹,而这将会导致其在不同环境媒介下表现出不一样的行为,甚至即使这些纳米材料来自于同样的母体材料[142]。纳米材料带给环境的风险不仅基于其数量或质量(浓度),也基于其独特的性质以及行为[134]。除了刚刚提及的方面,下列因素也是垃圾填埋处理ENMs时需要考虑的内容。
(1)ENMs的制造可能会产生需要专业化处理的、有毒性特征的纳米副产物或其他纳米废弃物。
Templeton等人[143]研究了单壁碳纳米管(single-walled carbon tubes,SWCTs)对甲壳类动物影响的实验,结果发现虽然原始纯化的SWCTs对这些测试种类没有任何有害影响,但其副产物(在电弧放电合成中产生的合成副产物)却对这些动物有潜在的有害影响。纳米材料副产物的实验带给我们很大启发,促使我们今后评估纳米材料对环境和人类健康危害性时考虑更多[143]。
此外,单一ENM的制造也可能产生其他具有不同危害程度或不同形式的纳米废弃物。例如有10种主要类型的多壁碳纳米管(multi-walled carbon nanotubes,MWCTs),可以通过5种不同的工艺技术制备而成(某些类型会含有不同程度的杂质),且通过使用3种不同的纯化技术及10种可能的表面涂剂(在实际应用中用以维持材料的纳米特性)来改变纳米结构的尺寸[121]。
(2)纳米产品的处理可能造成各种各样的风险状况。
对ENMs危害性的评估不仅应考虑其本身特性而且还应考虑其暴露在环境或人类中的程度。例如,某种ENM可能危害性比较高,但是如果其存在于产品中(很低或没有暴露的可能性)则更可能展现低的危害性。但是,某种ENM与其附着产品(比如防晒霜)的结合并不紧密牢固,则可能由于纳米材料(纳米二氧化钛,纳米氧化锌,富勒烯)自身毒性的不同而展现不同的毒性[132],或者是由于其在产品表面所结合的方式不同而毒性有所不同[144]。因此,针对此种情况,为了更好地处理ENMs必须充分考虑其与产品结合的程度、结合的方式以及它们使用或应用的特点。
(3)ENMs可以结合在污染物上,从而增加污染物的毒性,也可能加速污染物向空气、土壤或水中的迁移。
污染物对ENMs的吸附会导致毒性和转移速度的增加,在某些情况下,甚至可以增加污染物的生物利用度。He等人[145]发现,除了有机分子外,具有潜在毒性的金属离子也有能力吸附在纳米粒子表面,从而增加金属原子的转移速度和毒性影响(促进了ENMs在治理毒性金属污染物中的使用)。Gao等人[140]也发现了同样的问题,他们的研究结果表明如果汞吸附在ENMs上,一旦进入自然环境中,就会变得具有生物可利用性以及毒性。Cheng[146]与Yang等人[147]也报道了有机复合物比如多环芳烃(polycyclic aromatic hydrocarbons,PAHs)可以吸附在碳纳米管上,从而增加了PAHs的毒性。然而,也有一些例子表明ENMs可以降低附着物的毒性[148]。
一些ENMs由于尺寸较小以及沉降率较低,可以长期在空气或水中保持悬浮的状态,与同样材料的大尺寸粒子相比,它们的移动距离更大,移动范围更广[118]。根据ENMs与土壤的性质,ENMs可以保留在土壤粒子中或者通过土壤进入地表水[118],如果土壤中黏土含量高,则ENMs会更加稳定且分散性更好。然而,Lecoanet等人[150]认为ENMs表现出的移动行为区别很大。
(4)环境中ENMs浓度的增高可能通过不同的食物链引发长期的不良影响。
某些ENMs可以被生物有机体长时间滞留或摄取,且在食物链中不断地经历着生物降解或生物聚集过程,从而具有生态毒性,可引发长期的慢性影响[118,151]。对食物链产生毒性危害的有细菌、植物与多细胞水生与陆生有机体[117,126,152]。此外,一些ENMs的吸附能力以及渗透膜的能力引起了科研人员对有害化学物质在组织和细胞中移动的担忧[132]。虽然某些ENMs没有毒性,但如果与其他传统含有毒性物质的废弃物混合/反应的话,这些无害的ENMs就起到了木马的作用,帮助有害物质进入细胞[153]。然而,可以作为特洛伊木马的ENMs的数量依赖于ENM表面和其他物质表面分别与有害物质的结合能力的强弱[154]。
在对各种纳米产品、纳米废弃物与ENM制造过程产生的副产物进行处理时,应认识到不同的产品具有不同的危害性,其所含有的ENMs也具有独特的物理化学性质。对于ENMs与渗出液中其他污染物潜在的相互作用也需要进一步研究,因为这也许会对污染物的毒性或扩散有影响。在最糟糕的情况下,这些因素可能引发环境中污染物的扩散。对废弃物填埋管理关注不足会引发ENMs的排放,从而会导致土壤、表面与地下水源的污染[132],这种情况尤其可能出现在非工程化、不受控的填埋场。当前该方面的研究持续得到了欧盟、美国以及法国的资助。
填埋场现场填埋条件的变化可能会在很大程度上影响ENMs的行为,因而随着时间的推移,ENMs表面及反应活性均会有所变化[135]。填埋场长期保持无氧状态,然而,其他的条件,比如pH值,一般情况下会随着时间的推移而增加。填埋场也会采用机械方法通过压缩废弃物而减少其所占空间。此种情况下,纳米产品中ENMs的释放将可能在填埋堆中发生[119,122,135]。ENMs的最终结果与纳米粒子的移动性、自身的可降解性以及母体材料的可降解性有非常大的关系[155]。填埋场的物理化学以及水分条件可以影响母体材料以及ENMs的变化[114]。
有科学文献指出,取决于ENMs化学键的特性、位置和质量,一些ENMs易于降解和/或在填埋的条件下可以从纳米产品中释放出来。ENMs释放的难易程度依赖于其在纳米产品中的位置[156]。然而,这需要进一步研究加以证实。当前由美国主导的NanoRelease项目2(2 NanoRelease项目分4个阶段展开,依据上一个阶段的研究成果决定下一阶段的研究范围和方向。第1阶段参与机构众多,有美国环境保护局(US Environmental Protection Agency),加拿大环境研究与发展办公室(Office of Research and Development, Environment Canada),加拿大卫生部新兴重点处(Emerging Priorities Division, Health Canada),新物质评估和控制局 (New Substances Assessment and Control Bureau),美国化学理事会( American Chemistry Council), 纳米技术专家组(Nanotechnology Panel),美国化学品制造商协会及成员(Society of Chemical Manufacturers and Affiliates),美国国家标准技术研究所(National Institute of Standards and Technology),美国黏合剂及密封剂委员会(The Adhesive and Sealant Council),美国清洁用品研究所(American Cleaning Institute)。)就包含相应的内容,此项目旨在支撑对ENMs排放机理的研究以及提升对ENMs危害性的风险防范3(3 国际生命科学学会(International Life Sciences Institute)“NanoRelease项目”www.ilsi.org/ResearchFoundation/RSIA/Pages/NanoRelease1.aspx。)。德国也有与此相关的项目,包括FRINano4(4 FRINano项目是一个研究并建立即时测量技术的项目,目的是用于量化和表征颜料纳米粒子,这些纳米粒子在风化和/或在机械外力下可从涂料或塑料中释放出来。www.vdmi.de/englisch/topics/nano.html。), CarboSafe5(5 CarboSafe 项目是一个研发准确判断CNT-基产品排放纳米粒子速率的可靠测量技术的联合计划。此项目旨在识别碳纳米管的生态毒性以及在新的测量技术帮助下精确估计相应的潜在风险。www.nanopartikel.info/en/projects/completed-projects/innocnt-carbosafe。), CarboSave 与CarbonLifeCycle6(6 CarboLifeCycle 项目是一个关于纳米安全方面的研究项目,该项目强调碳纳米材料的生态毒性的考量、测量工程的发展、测量技巧的提高,以及生产、加工、利用和报废过程中泄漏程度的量化。)。
一般来讲,ENMs如果被牢固地束缚在固体纳米产品(例如汽车部分、存储芯片等)内部,暴露的程度几乎没有或者很低(暴露于环境或活的有机体)。然而,即使ENMs与其附着的产品结合再牢固,填埋场苛刻的环境条件,比如低pH与强还原性条件(归因于无氧环境),也有可能促使ENMs从聚合物中释放出来[135]。塑料/树脂/聚合物/金属产品中包含的物质,比如在建筑废弃物中发现的物质,在机械外力的挤压[122,138]和/或与性状特别严苛的渗出液接触的情况下,也可以排放到渗出液中[119]。
在对复合物中碳纳米管潜在排放的研究中,Nowack等人[122]讨论了如果填埋碳纳米管复合物,由于其自身的生物降解性,其缓慢分解且将ENMs排放到渗出液中的可能性(或通过复合物风化而形成的灰尘)。然而,聚合物母体在现代化的填埋场中排放碳纳米管的速度可能极其缓慢。相比之下,在非现代化的填埋场中可能会有大量的碳纳米管的排放[122]。
相反地,悬浮液中松散的ENMs状态表明其可能有非常高的暴露度[132]。例如,化妆品、防晒霜、洗发产品、废水生物固体以及纳米材料制造废弃物中ENMs的排放符合上述结论。一旦纳米材料排放到渗出液中,渗出液中的组分将会显著影响材料的处理结果[119]。Boldrin等人[114]考虑到化妆品中有大量的ENMs需要处理,因而针对防晒霜中的二氧化钛进行了暴露评价,并根据其潜在的暴露风险将其划分到“中等级别”。然而,当纳米粒子从最初的产品中排放出来后,许多纳米粒子易于被修饰或者说是发生变化,因此在处理ENMs时需要充分考虑这一点[157]。ENMs也会发生聚集形成更大的粒子从而失去特有的纳米性质。
产品中的ENMs如果处在产品的表面或者在产品中以松散的状态存在,则可能会对纳米粒子的排放有一定的影响。虽然个人护理产品或者其他分散在液体形式中的ENMs问题更大,更易于与渗出液的化学组分发生反应[119,135],但填埋场的条件(化学的或物理的)也会促使纳米粒子从固体废弃物产品中排放,也需要予以考虑。需要对ENMs在不同环境条件下的变化进行更深入的研究,包括精确预测填埋对其危害性的影响的研究。
研究表明渗出液中的有机物质会影响ENMs的稳定性、聚集以及移动,也有研究探讨了pH值及其他因素对ENMs可溶性和聚集性的影响。然而,渗出液(胶体体系)是非常复杂的,本报告并不试图对该领域进行更深入的科学分析。一些科学项目开展了对ENMs排放到环境中后会发生哪些变化的研究,这些项目有FP7 NMP (纳米材料计划),NanoSUN (可持续纳米技术)和NanoMILE (纳米材料与生物体和环境相互作用的机理:安全纳米技术通用框架)。
对ENMs与pH值之间相互关系的研究得知,水中纳米粒子的稳定性依赖于其化学结构、液体的pH值和温度[137]。研究显示,水的碱性越强,富勒烯(C60)聚集就越少,也就是随着水中pH值的升高C60聚集体的直径就会降低。然而,Labille等人[158]对防晒霜中二氧化钛老化进行了研究,二氧化钛的老化受溶液pH值、离子强度与天然有机物质浓度的影响,研究表明胶体易于聚集并从水中析出[158]。此外,Gao等人[140]讨论了ENMs吸附污染物是如何依赖pH值的。pH值对固体吸附的影响更大,但是在复杂介质中,如渗出液,有许多别的吸附物会与ENMs展开竞争,因而pH值不再是主要的影响因素[136]。低pH值时,金属ENMs会带有正电荷,高pH值条件下,其会带有负电荷。在特定的pH值,ENMs会变成中性,因而此点被称作等电点(isoelectric point,IP),粒子倾向于聚集[159]。另外一个需要考虑的因素是ENMs经常会带有有机物质涂层,目的是保持粒子以悬浮状态存在于产品中,而这会对于粒子的聚集行为有所影响[160]。
有多项研究报道了渗出液中有机物质间的相互作用及对ENMs移动的影响。填埋渗出液中的有机物,尤其是时间较久的填埋物中产生的有机物,比如腐殖酸和富里酸,据报道可以稳定ENMs[161-163]。稳定性的提高,减少了粒子的聚集,因而也会增大纳米材料的移动性[164]。Jaisi等人[165]与Lozano和Berge[119]报道了单壁碳纳米管的移动性由于腐殖酸的存在而增强。类似地,Lin与Xing[163]也报道了鞣酸提高碳纳米管移动性的研究内容。Saleh等人[162]则报道了天然有机物质增强单壁碳纳米管移动性的研究内容。中佛罗里达大学主导的研究表明,腐殖酸可以增强渗出液中氧化锌纳米粒子的移动性[113]。Lozano与 Berge[119]的研究认为,甚至在高离子强度,腐殖酸对材料的聚集/合并也制造了空间障碍,而这可能会帮助纳米材料脱离废弃物。
一般来讲,pH值是影响ENMs在溶液中聚集的众多因素(诸如离子强度、温度、天然有机物、具体的ENMs性质等)之一,但是其可以在不同状况下对纳米粒子的聚集产生促进或抑制作用(与其他因素相结合)[152]。腐殖酸及其他有机物对ENMs影响的研究结果表明,它们都可以通过稳定ENMs的存在而减少纳米粒子的聚集,从而减少沉淀。相反,如果腐殖酸和鞣酸对于背景物质的亲和性强于ENMs,也可以限制ENMs的移动[136,166]。填埋处理过程中,ENMs沉积的减少将会增加不同类型ENMs的移动距离。然而这并不是权威性的结论,还需要进一步的科学调查。
Holden等人[117]讨论了因ENMs导致的环境中细菌多样性的降低而带来的对生态系统和人类健康潜在负面影响的问题。多项研究工作已经展开,旨在调查填埋处理方式下ENMs的抗菌特性;另外一些仅作为比较之用的研究讨论了ENMs的抗菌性如何影响处理废水所用微生物功能的问题,尤其在生物处理厂[117,167]。在某些国家,填埋渗出液主要由废水处理厂来治理,因此渗出液中的ENMs可能会对治理过程中的有效性产生非直接影响。
通过不同的机理,比如形成活性氧(reactive oxygen species,ROS)与破坏生理代谢过程,ENMs展现出了抗菌性[151]。Yang等人[123]曾进行过一项有关ENMs影响的专项研究,即纳米银对垃圾填埋场中微生物作用的影响。虽然纳米银在低浓度时不会对生物气体的产生有影响,但当纳米银的浓度达到10 mg/kg时,就能够抑制生活垃圾产生甲烷和其他生物气体。中佛罗里达大学的另一项研究表明,氧化锌和二氧化钛纳米粒子与时间较久的渗出液接触时,不会对其无氧或有氧过程产生抑制作用,这一切要归结于较低的锌浓度[113]。
也有研究结果认为,许多金属ENMs的抗菌性会极大地影响废水处理的效果,这是因为ENMs的存在将会降低微生物功能,导致一些传统的化学和生物污染物没有经过这一环节的有效处理就被排放掉[131,132,137,167]。然而,这种现象仅在ENMs浓度较高时才会发生。Hou等人[168]的研究结果表明,纳米银(本文中指纳米银粒子)的浓度在0.5 mg L-1时,不会对活化污泥过程中铵的去除效率有明显影响。Yang等人[123]认为,污水中纳米银的银离子的排放会抑制硝化作用(细菌将NH3转化成硝酸盐),而且氧化锌和二氧化钛在高浓度时能够降低氮和磷的去除效率。硝化作用是就地填埋生物处理系统中治理渗出液的一个重要环节,目的是通过微生物(细菌)消除可溶性的有机污染物[169]。
根据以上信息可知,ENMs,尤其带有金属或金属氧化物纳米粒子的ENMs,在高浓度时可能会抑制填埋渗出液处理系统(与污水)的微生物处理作用,尽管还存在其他会影响微生物功能的变量,这些变量包括渗出液中不确定的组分如何与ENMs发生作用、它们的浓度、有氧还是无氧条件,以及ENMs在变化前后是否会展现出抗菌性。此领域还需要更深入的调查研究。
现代化的填埋场中会使用合成膜作为隔离层将填埋物与周围的环境隔离开来。紧实的黏土也可以单独被用来作为物理隔离物,但现在作为第二层防御与合成隔离物组合使用。有关ENMs穿透填埋隔离物的研究一直在进行,目前只是缺乏结论性的研究成果[134]。相应的研究团体有美国的东田纳西州大学与环境研究和教育基金会。
Siddique[170]最近的研究成果表明,构造和设计合理的填埋场能够在相当长的一段时期内(大约100年)显著限制纳米粒子进入到环境中。纳米废弃物管理部门(NanoHouse)主导的一项实验利用油漆纳米粒子悬浮液评估了防渗膜的性能。研究结果表明纳米粒子无法穿过该膜,这项实验的设计对应于在实际条件下土工膜可以保持12年以上的有效作用[171]。
然而,另一项研究表明ENMs如果被放置在生活垃圾填埋层的底部,那么它们就可以穿过隔离层,尤其是一些特别接近底部的ENMs[119]。渗出液是可移动的水相混合物,由于其可以进入到周围的环境中,因而会对人类的健康带来一定的危害[113]。
合成膜隔离层可以有效防止ENMs的渗出,相关内容目前正处于研究中。然而,还需要开展更多的研究工作,尤其对于一些老旧填埋场或者不受控的仅仅依靠自然能力进行处理的地方,需要对ENMs通过黏土隔离层的相关问题进行研究。
渗出液的治理可以采取一种或多种方式,比如通风、沉积、沉淀池、过滤、紫外线以及生物和/或化学处理。这些治理方法实质都是通过沉降固体物、调整pH、增加氧气与分解来达到避免环境及人类健康受到危害的目的。渗出液治理系统的有效性很大程度上依赖于ENMs的性质以及它们在填埋场环境中的行为表现。对此,需要考虑3点:(1)ENMs是如何与渗出液相互作用的以及作用后它们的移动性和/或毒性是如何增加或减少的;(2)隔离层的完整性与性质以及阻止ENMs的能力;(3)ENMs对治理技术有效性的影响如何。
虽然对废水处理技术去除ENMs的研究结果已经见诸报端,但当前缺乏的是对就地填埋渗出液治理系统阻止和/或去除ENMs的具体研究细节。本文对此进行了简要的描述,目的在于推断治理技术对填埋渗出液治理系统的可能影响。在对废水处理厂ENMs的研究中,通常键合有机物的纳米粒子最终会被沉降析出;其中一些自然而然地与其他纳米粒子聚集在一起,从而提高了沉降效率;还有一些与有机污染物键合,另一些粘合在其他可选择的表面上[137]。
研究人员发现传统的废水处理厂可以从废水中有效去除诸如纳米氧化银、纳米氧化锌、纳米氧化铈、纳米二氧化钛(Ag0、ZnO、CeO2和TiO2)等ENMs;而ENMs大部分(>90%)都聚集在了污泥或生物固体中[172]。此外,除了纳米二氧化钛,银、锌和铈的矿物学特性会因氧化、还原以及溶解而发生变化。而这会导致ENMs发生改变,从而使它们在不同阶段展现出与最初的ENMs不同的特性[136]。 Kaegi等人[157]发现,吸附在废水生物固体上的纳米银积累到一定程度后会经历化学过程,变化成硫化银 (Ag2S),然而硫化银的毒性相比其他形式的银却低很多。Kaegi等人[157]同时也认为,还需要更深入的研究以确定ENMs上其他类型的表面覆盖物是否会稳定纳米银或其他废水中的ENMs。Nguyen MD[173]的研究结果表明,纳米氧化锌(ZnO)与纳米氧化铈(CeO2) 通过抑制生物气的产生会影响厌氧消化过程,并且生物固体中ENMs的毒性可以抑制细菌繁殖、植物的种子萌发以及根茎生长。Barton等人2014年利用实验方法研究了ENMs与细菌群落的相互作用。最初的ENMs转成了新的材料例如草酸铈或硫化银或磷酸氢锌(ZnHPO4),而新的材料将不再与最初的ENMs具有同样的生物活性[174]。
直接将废水处理厂中的ENMs去除效率与填埋渗出液的处理进行比较是非常困难的,因为渗出液是水相流出物。然而,ENMs会与渗出液中有机物和细菌结合在一起的论点也不太能让人信服。在生物渗出液治理环节中,由于沉降固体的聚集,残余的污泥中将会出现ENMs的身影。最近的研究表明,在一些案例中,生物固体中的ENMs甚至其变形物都能被成功去除和捕获。然而,如果污泥再次使用或送往填埋场,污泥中的ENMs仍然具有排放到周围环境中的可能性[137,152,172]。为了妥善管控生物固体,识别含ENMs的生物固体的风险非常必要。而这需要更多的思考和深入的研究;尤其是填埋场里含有ENMs生物固体的处理。
用于ENMs就地填埋处理方式的最优实用技术(BAT)尚未得以确认,然而相应可能的技术正在研发。虽然并不是所有的ENMs都有毒性,也不是所有的ENMs都需要专业化处理,但阻止那些有危害的ENMs的排放是必须的。首先根据危害级别对ENMs进行分类,从而识别出最佳处理方法,也就是逐项处理的理念。研究纳米废弃物的处理需要了解其全部性质,不仅仅是化学的,也包括物理和生物性能[115]。有关ENMs的处理方法已经有所研究,同时含有ENMs的工业废水处理系统也正处于研发阶段,这可以提供一些有用的信息,从而使技术得以改善并有效应用或与现场填埋处理系统相结合。
最近,一项由法国国家科研署资助的项目NANOSEP显示,包括絮凝、膜渗透与浮选等在内的治理技术在去除ENMs方面非常有效。此项目同时还表明絮凝和膜分离的联合使用同样十分有效。Lui等人[152]也识别与评估了几种处理技术,展示了影响废水中ENMs成功去除的几个因素:(1)凝结与电凝结过程;(2)浮选过程;(3)过滤过程;(4)生物过程;(5)其他ENM分离过程。Lui等人[152]同时指出,仅使用一种技术去除复杂母体中所含的ENMs是比较困难的,通常选择不同的技术联合使用才能达到较佳效果。Westerhoff等人[172]讨论了下列内容及其效果:(1)纳米材料的膜分离;(2)生物处理过程中纳米材料的生物变化;(3)持续式废水处理系统的纳米材料去除。DiSalvo等人[137]指出,水相(或废水),比如渗出液,其中的纳米粒子的去除可以使用纳米过滤或反渗透技术。
欧洲NANOFLOC项目将重点放在了新技术开发上,新技术试图以电絮凝破坏纳米悬浮液的稳定性以及促使带电荷纳米粒子的聚集为基础构建完成。NANOFLOC同时也探究了其他可行方法,比如凝结和沉淀、浮选、磁分离(仅适用于磁性粒子)或零价铁应用。目前,上述方法没有一个可以单独使用并且表现出高效性。
想要有效处理危害性固体纳米废弃物,可以将ENMs牢牢锁定于固体母体中,也可以将其包裹于其他致密紧实的包含物中[175]。其他固定高危害性废弃物的方法,如玻璃化,目前被广泛应用于核工业废弃物的研究[176],此类方法也可以用于探究高危害性纳米废弃物的处理[177]。Bystrzejewska-Piotrowska等人[115]建议WCNMs不宜与水接触(可能为了降低移动性)。针对土壤或水中的纳米粒子污染物也出现了新的生物治理技术,例如生物萃取技术(使用真菌去除污染物)[178]。
如果能利用最好的管理实践对有害ENMs进行危害性分类、标记和分离,那么最优实用技术就会有效。对于水相中ENMs的处理,比如渗出液(可能含有非危害性和危害性ENMs),很多技术在去除废水中的ENMs方面表现出了有效性。用于工业(或其他)目的的方法当前正在试验中,将来也可能会用于废弃物处理领域,至于去除渗出液中的ENMs还需要与其他先进处理系统组合使用。
2013年,OECD采纳了一项有关人造纳米材料安全测试与评估的建议[179]。这等于接受了“传统化学品测试与评估一般情况下也适用于评估纳米材料的安全,但为了符合纳米材料的具体特性可能需要一些修订”[179]的观点。因此,诸如欧盟REACH法等法规在多数情况下适合于解决ENMs危害性问题,尤其是经过针对纳米材料的修订后。同样,Breggin与Pendergrass[180]认为当前的美国法规也能覆盖ENMs的治理。然而,研究文献也表明,对于一些ENMs,当前表达毒性的体系可能存在一些局限性,需要对控制范围以及废弃物管理方法做出一些适当的调整。目前,环境立法的焦点在大规模的化学品上,相应的风险考虑也是在于暴露度以及单位体积表现出的危害性或毒性。科学研究表明一些ENMs的毒性与其形状、尺寸、表面活性和表面积相关[132,180,181]。
本领域中的知识空白可能会限制当前管理手段的有效性。关键的知识空白点包括ENMs危害性表征、填埋场环境下对ENMs行为表现的了解以及毒性方面数据的量化。例如,对制造过程中产生的纳米废弃物副产品的处理很可能比正品转变成废弃物后的处理更加严格。由于当前没有足够的毒性数据以及移动信息和适宜处理技术,对纳米技术制造过程中产生的副产品的毒性所带来的风险没有较为充分的应对措施[132]。对有危害性的ENMs进行识别和标记对科学处理非常重要,这将极大有助于危害物的分离和回收,从而阻止其进入到生活垃圾填埋场[115]。然而,如果想保证产品标记的有效性,必须给出纳米材料的官方定义以及分类范围。
对于在处理过程中可能展现出危害性的消费类纳米产品,标记以及指出适宜的处理建议可能会有利于这些产品报废后的管理,这样需要特别处理的消费类纳米产品即可采用与处理其他家庭危害废弃物相似的办法。纳米产品比如防晒霜可能对于消费者没有任何危害,但是由于其可能在填埋的环境条件下或废弃物处理流程中与其他材料相互作用或者自身发生降解而展现出不同程度的危害性[132]。此部分内容还需要更深入的研究和更多的考量。
由于不清楚众多公司使用或存储可回收和不可回收ENMs的计划,因而对相应控制措施、法规或其他废弃物管理条款的制定或实施带来了一定的挑战。为了能够充分评估ENMs使用所带来的可能风险,相应的公司应该提供关于ENMs数量和性质的基本信息,以及含有纳米粒子产品的使用期限信息[182]。不过,纳米废弃物的生产者也许还无法就产品的处理、储藏及排放措施向产品的拥有者和使用者提供充足的信息,使他们能够正确管理废弃物[180]。
为了降低ENMs从填埋场中排放到周围环境的风险,分离、回收、充分的填埋设计和操作、有效的渗出液处理技术以及必要时专业化设备设施的安装等方面都需要不断完善,做到整体协同考虑。ENMs的识别、分类以及标记有助于废弃物管理方法的实施以及适宜技术的选用。从严格防止危害性ENMs进入生活垃圾填埋场的角度考虑,修订并明晰当前的法规以及废弃物管理办法也许是有必要的。
目前公认的是,虽然有关ENMs及其在填埋场的最终结局及表现的认知水平在不断提高,但为了对各种各样的含纳米材料废弃物进行更加有效的管理还需要继续开展更加深入的调查研究。然而,最近的研究结果反而提出了更多复杂的需要解决的问题。有证据表明,在填埋场的环境里一些ENMs从含纳米材料产品以及其他纳米废弃物中排放出来。因此,我们可以合理地推断,目前填埋场中含有ENMs,如果ENMs能够穿过填埋隔离层(尤其从未受控制的填埋场)或通过垃圾渗出液排放,那么填埋也是ENMs进入环境的一条途径。调查研究中的第二条途径包括ENMs通过填埋气迁移至环境。
随着纳米技术行业的快速成长以及纳米材料的广泛使用,填埋场里的ENMs数量将会显著增长。产品释放ENMs很可能是在典型的垃圾填埋条件下,尤其是液体废弃物或其他比较松散地包含纳米粒子的产品。填埋场比较特别,有着复杂的环境条件,ENMs的行为表现及排放受pH值、无氧条件、渗出液组分以及许多其他因素的影响。例如,渗出液里的有机物通过阻止ENMs的聚集和沉淀来提高其移动性。物理来源比如磨损和压缩也可以促进填埋场里ENMs的排放。
由于独特的物理化学性质和特征,比如尺寸、形状、表面积和化学活性,ENMs与其他已知的污染物不同。处理过程中,不同阶段的纳米材料由于具有不同的形式而具有不同的危害性。一些ENMs能键合吸附在别的污染物上,从而导致这些污染物的毒性和移动性提高。如果考虑到与填埋渗出液的相互作用,ENMs的这些独特性质就成为了大问题。最糟糕的情况是,已经含有各种污染物的渗出液可能会变得更具毒性,更具生物亲和力,并且会将其他污染物转移至填埋场之外的生态环境中。然而在填埋场或周围环境中ENMs可能会发生变化从而不再保留原有的特性。反过来,这些变化将会影响ENMs在环境中的迁移、归宿和毒性。
填埋场中ENMs的抗菌效应尚未得到很好的研究;然而,当菌落被用来分解污染物时,高浓度的ENMs可以降低渗出液治理的效果。虽然科研人员正在对ENMs能否穿过填埋隔离层进行研究,但目前还没有结论性的结果见诸报端。当前人们关心的主要问题是渗出液中ENMs的处理,这些渗出液会被收集起来通过废水处理环节进行处理,亦或被直接排放到周围的环境中(无论是否经过现场处理)。
虽然没有更多有关处理渗出液ENMs的最优实用技术的具体报道,但是正在使用或者正在研究的技术在去除ENMs方面都展现出了不同的效力。
ENMs独特的性质给当前的管理体系和法规是否能够充分识别和解决ENMs带来了挑战。具体来讲,ENMs在不同状态下能够展现不同的危害性。在当前的法规下,ENMs可以得到处理;然而,为了避免给公众、商业、保险以及投资人带来远期的不利影响,需要对相关法规进行明晰与修订,以充分指导相关行业和监管者控制ENMs所带来的危害。
虽然有关ENMs的科学研究早已启动,且近来也取得了一些成绩,但远远不够,我们依然需要努力去提高对ENMs的认知,依然需要努力去寻求更加有效的解决办法。下列内容值得我们予以关注。
(1)发展用于识别环境介质中ENMs及区分常规化学品的分析化学测试方法。
(2)对填埋场中化学环境过程的理解及相关问题的表征和量化。
◆ 识别ENMs的类型、数量、危害等级、暴露可能性,评估含有ENMs的产品的风险,包括处理过程中的产品及废弃物中含有ENMs的产品。
◆ 发现适用于其他基质(比如水、废水、气体)的现代分析方法,将这些方法应用于渗出液和填埋气中ENMs的浓度分析以及填埋方式下ENMs的迁移及最终归宿的研究。
◆ 理解填埋渗出液中ENMs和典型污染物的协同效应;特别关注渗出液中关键的污染物,研究ENMs对这些污染物毒性、生物可获得性及转移的影响。
◆ 理解ENMs在填埋环境(渗出液里)中的降解和变化过程以及降解产品的影响;纳米产品和纳米废弃物中ENMs的影响和排放。
◆ 判断ENMs是否从填埋物表层或通过填埋气排放到空气中。
(3)理解当前填埋方法和技术的有效性和局限性。
◆ 理解ENMs对就地填埋处理系统中微生物性质的影响以及ENMs对渗出液处理系统的其他可能影响。
◆ 识别关键ENMs穿过填埋隔离层和通过渗出液处理系统的情况,并判断传统方法和其他技术对其治理的程度(与研究废水处理工程中ENMs类似)。
◆ 判断当前应用于废水处理系统中的最实用技术在处理填埋渗出液中ENMs的适用性。
◆ 开发从生活垃圾填埋场中分离危害性ENMs及处理含危害性ENMs废弃物的有效方法(亦即充分地处理含有ENMs的残余废弃物比如生物固体或灰,而不是简单地将它们转移到填埋场)。
(4)理解用于废弃物管理的未来ENMs分类系统的适用性。
◆ 核查分类、标记与分离等步骤对通过专业化危害废弃物填埋(或其他处理过程)处置有害纳米废弃物或含有危害性ENMs废弃物的有效性,以此确保处理的适宜性、充分性和安全性。
本章对ENMs的知识现状及其在废水处理工艺中的行为进行调查,以期确认未来的研究方向。本章着眼于城市现行污水处理工艺,首先对ENMs在废水处理厂中的存在性进行调查,进而对ENMs在活化污泥中的潜在沉积与聚集进行分析,研究其在废水处理过程中可能发生的转变及用以预测说明此类变化的模型。本章还对携带ENMs的污泥应用于农业可能带来的风险进行了讨论。文章最后对该领域的知识空白进行了识别,并指明了未来的研究方向。
本报告对ENMs的知识现状及其在废水处理过程中的行为进行了研究,以期为未来的研究探明方向。
本章首先介绍废水处理工艺并对人工纳米材料在废水处理环节中的存在性进行探究。而后通过模型分析了活化污泥中ENMs的潜在滞留、聚集及沉淀行为。同时对含有ENMs的污泥应用于农业可能带来的风险进行了讨论,并对该领域的国际研究现状进行了描述,最后强调了知识空白及今后需重点开展的研究工作。
污水处理厂收集来自城市及工业的废水。城市废水源自人们的日常生活(如厕、洗浴及餐具清洗等)。由此产生的污泥量很难测量。不过法国环境与能源管理局(French Environment and Energy Management Agency,ADEME) 2004年发表的报告对数据进行了确认,数据表明农业应用占据了相当庞大的数量(表7.1)。
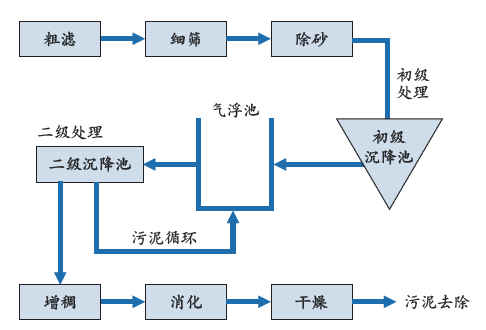
大多数城市废水处理厂是以生物处理工艺为基础的处理厂,有时也会采用物理/化学方法(絮凝、氯化等)。图7.1展示的是一城市污水处理厂所用工艺的各个环节。
首先将较大的杂质去除:粗滤+细筛+除砂。
生物处理在气浮池中进行(加入空气),随后污泥沉淀并被循环回到气浮池顶部。未被回收的污泥经过增稠进入消化环节,即在厌氧反应器中稳定有机物质(去味)、降低毒性(阻挡金属及病原体)、分解有机碳、降低需要再处理污泥(干物质)的量;干物质从35%提高到40%,挥发性物质则从40%提高到50%。
生物处理利用异养细菌减少有机污染,因为异养细菌以有机物作为其能量来源。通过细菌作用还可以吸附金属元素,聚集一级筛检工艺未能去除的微粒。
污水生物处理厂占了液体废物处理厂的大多数。生物处理也被称作活化污泥处理,即利用多种细菌来分解有机污染物(杀虫剂及医用残留等),阻隔金属及类金属物,对污水进行除氮化。这是一个复杂的工艺过程,同时还涉及生化反应釜和物理工艺如聚集及沉淀等反应。活化污泥是一种复杂的混合物质[184],由细菌聚集体组成(~500 μm),而细菌聚集体又是由微团聚体构成(~10 μm)[185]。直径约2.2微米的分形结构使得水无法进入聚集体的中心。而在有氧反应釜中细菌的多样化可保证反应的广泛进行。
除细菌以外,聚合物(蛋白质及多糖)也承担对不同污染物的俘获工作。
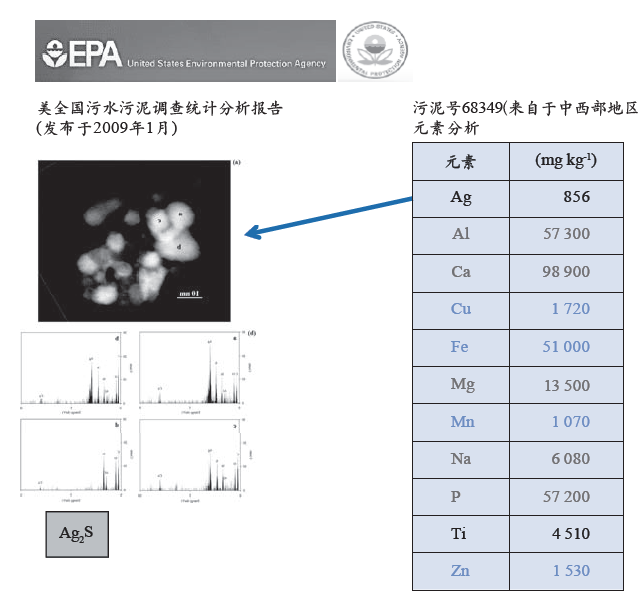
由美国EPA资助的一项研究(全国污水污泥的调查统计分析报告,“Targeted National Sewage Sludge Survey Statistical Analysis Report”,- EPA-822-R-08-018 - April 2009)证实,来自城市污水处理厂的污泥中存在相当浓度的银(Ag)或钛(Ti)(图7.3)。对EPA研究样本的后续调查发现了硫化银纳米粒子的存在[188]。这些硫化银纳米粒子来自于Ag被氧化生成Ag+,这些Ag+与S结合生成热力学稳定的Ag2S(图7.3)。
当今ENMs已存在于日常消费品中,如化妆品、涂料及农产品等领域[187],OECD各成员国的污水处理厂是人类活动产生的废水的重要处理机构。2010年,超过70亿立方米的城市污水及雨水经过了污水厂的处理。详见表7.2。
在废水处理厂的初始阶段,废物中纳米粒子会经历聚集、沉淀等变化,有时某些纳米粒子会彻底改变,导致其在污水中甚至污泥中的浓度发生改变,而污泥将被送至下游进行焚烧、储存或应用于农业。因此在对城市及工业废水进行处理的时候,了解并预知其中纳米粒子的去向至关重要。以含有表面功能化的ZnO及TiO2纳米粒子的化妆品为例,人们在污水处理工厂排放的水中发现了该种微粒[190-193]。
近期学界涌现出许多对废水处理厂活化污泥中纳米粒子或纳米材料的变化及其影响方面的研究。研究最多的纳米材料包括金属银纳米粒子,其次是ZnO、TiO2、CeO2、SiO2及碳纳米管。
污水中的纳米材料如TiO2、Ag0、CeO2或Cu经过一级和二级处理后得到了有效的去除[190,194-198],超过80%以重量计的纳米粒子进入固态污泥当中。造成这种结果的机制包括纳米粒子和细菌的异体聚集,外加吸附性以及与生物聚合体的相互作用[197]。也有学者提出纳米粒子与生物有机体相互作用发生的物理化学变化也起着重要的作用[199]。纳米粒子的多样性、表面的功能化以及其独特的表面积都会在动力学以及数量方面影响它们的去除效果[190,194,199,200-203]。一小部分纳米粒子最后将会从处理厂流出进入地表水。
对废水处理过程中纳米微粒的稳定性研究[204]表明,二氧化铈对蛋白质特别是肽类有亲和性。Zeta电位的改变增强了纳米粒子的稳定性。对Ag0的类似研究表明,表面功能化[194]使得纳米粒子非常稳定不易被有效去除,而非表面功能化的纳米微粒则更容易出现在固体物中。
纳米粒子可迅速与废水中的其他粒子相互作用从而发生变化,例如Ag0的氧化及硫化[195,205-207]。硫化改变了纳米颗粒原来的反应活性,这是由于硫化使其溶解度降低且毒性减弱,而这都要归功于Ag2S纳米粒子不仅热力学稳定而且是非杀菌的[208,209]。对ZnO纳米粒子的研究也得到了类似的结果[210]。数据表明ZnO在处理过程中迅速转化为ZnS,硫化锌在堆肥中溶解,其部分Zn2+以磷酸锌形式形成沉淀,同时还可与铁的氢氧化物结合。
最近的一项研究[201]是在试验性反应装置中使活化污泥中的非功能化和功能化CeO2纳米粒子在有氧条件下与低浓度柠檬酸分子(~1mg/L,一月后)接触,表明Ce(Ⅳ)被还原成了Ce(Ⅲ),而且以Ce(Ⅲ)PO4的形式沉淀了下来。非功能化和功能化CeO2纳米粒子的反应表现是不一样的。非功能化CeO2的反应速度较快,其在菌落中可达30%,24小时后,表面被柠檬酸盐包裹的CeO2在菌落中为12%。这个结果说明纳米粒子与细菌膜的直接接触在金属氧化物纳米粒子的物理化学变化过程中起着非常重要的作用[211,212]。有机物或矿物质分子修饰的表面功能化粒子,其反应动力学及毒性都有所降低。因此,表面功能化纳米粒子减缓反应动力(如氧化还原反应),对废水处理工艺造成不利影响,但该报告同时提出表面功能化纳米粒子(如果修饰稳定的话)可以降低毒性,这对于工艺将会起到正面的作用。文本框7.1对以上内容做了归纳。
文本框 7.1 ENMs的物理化学改变
污水处理厂处理纳米材料的过程所可能发生的化学反应是必须要考虑的重要内容,比如通过还原(比如CeO2)或氧化(比如Ag0) 溶解性增强的情况。纳米材料的化学变化往往伴随着沉淀的发生比如Ag2S或CePO4,而它们都是热力学稳定性较高,且毒性比变化前减弱的化合物。而当今产品中纳米粒子的表面功能化会通过限制与细菌聚集体的接触减缓该变化的反应速度,且使得粒子较长时间内保持原始的氧化或还原状态。
科研人员调查了下列因素:
◆ 溶解氧需求的变化
◆ 硝化和去硝化作用
◆ 污泥处理过程中对厌氧条件下甲烷生成和挥发性有机酸的影响
◆ 生物需氧量
◆ 细菌多样化
◆ 胞外聚合体(特别是蛋白质)化学性质的变化
◆ 细胞消亡
◆ 纳米粒子与细菌相互作用的机制
◆ 污泥沉降对污泥结构变化的影响
然而不同科研人员的调查并没有获取较为一致的结果。例如:
◆ 有研究[213]表明,在对去硝化过程的影响上,与银离子相比,Ag0纳米粒子被柠檬酸盐修饰后的浓度达到~2×10-6时可以达到最大的去硝化抑制效果。而此数据完全与Kiser等人[194]在2010年的实验观察结果相反。有研究人员[214]指出,当银纳米粒子的浓度未超过40mg/L时,其对厌氧消化的影响可以忽略不计。
◆ 为了评估多壁碳纳米管对产生胞外聚合物及其呼吸的影响,科研人员对位于麻省的气浮反应池中的活化污泥进行了研究。结果表明抑制作用取决于碳纳米管的浓度,其浓度必须大于> 0.64 g/L时才起作用[215]。
◆ 一篇关于金属纳米粒子对厌氧消化影响的重要综述[214]认为,无氧条件下TiO2、Ag0、ZnO对细菌的多样化没有或仅有极低的影响。
◆ Z. Liang 等人[216]在2010年发表的有关于Ag0的研究论文与上述观点有部分的不一致,该论文研究结果表明,硝化细菌菌落随时间逐渐减少。
文本框7.2为上述内容的摘要。
文本框 7.2 不同处理阶段的操控
对不同处理阶段操控的研究依然处于初级阶段。根据纳米材料的数量及其表面状况、在有氧或无氧条件下菌群的变化等还需要更加系统的研究,这是因为这些菌群是上述反应的开启者。一些实验结果包括高浓度时的研究结果似乎并不能完全令人信服。
当前的数据表明绝大多数污水处理厂中的ENMs都存在于生物聚集体中,随后部分生物聚集体被循环至肥料中。我们知道一些纳米粒子诸如ZnO、Ag0、CeO2都会发生变化,而与变化相关的性质(氧化还原性+溶解性+沉淀性等等)不仅仅依赖于粒子的表面功能化处理,还依赖于其与生物膜的直接接触,比如一些展示出良好电子转移能力的生物种类。然而,这并不适用于最常见的案例:TiO2。TiO2溶解性特别差,其可以产生较强氧化能力的光催化活性依赖于其尺寸大小以及特定矿物面的延展[217,218]。
Barton等人[202]与美国纳米技术环境应用中心(CEINT)、法国国家科学中心、法国纳米材料安全生态设计、教育、研究与开发卓越实验室(SERENADE)合作,系统地测量了杜哈姆(北卡罗莱纳州)城市污水处理厂中与生物固体物结合的纳米粒子的数量,在明确的接触时间下(接触时间为1小时):
◆ ~90%的CeO2、ZnO和TiO2纳米粒子与细菌聚集体结合在一起
◆ ~60% 的Ag0纳米粒子与细菌聚集体结合在一起
表7.3提供了更加详细的数据。
表面功能化与没有功能化的纳米粒子的表现是不一样的。低浓度时(<10×10-6),没有功能化的纳米粒子在细菌聚集体中的规模远超功能化的纳米粒子。简单来讲,反应过程中能量的消耗促进了纳米粒子与细菌聚集体的结合,这是因为二者的混合将会增加能量的供给。
分配系数是一种用来评估在给定初始浓度的情况下,一定的固定接触时间后被固相滞留的可溶性物质数量的简单数学方法:
γ =$\frac{滞留纳米材料(mg)/生物固体物(mg)}{清液中的纳米材料(mg/L)}$
上述公式表明,分配系数的大小依赖于:(i)与化学稳定的纳米颗粒(TiO2)相比,当纳米颗粒存在表面功能化的情况下,导致其分解和溶解(比如Ag0, CeO2)、甚或具有不变氧化态的纳米颗粒(如ZnO)溶解的还原或氧化反应的可能性。(ii)接触时间,在有氧或无氧的状态下接触时间可以保持1分钟至60分钟。例如小于10纳米的Ag0粒子其γ值随时间的增加而降低,原因就是小粒子比大粒子拥有更快的溶解动力学性能[207]。
另一方面,无论是在一级阶段(有氧)还是在二级阶段(无氧),TiO2纳米粒子的γ值随时间的推移都展现出了稳定的增长。
CeO2纳米粒子的γ值随时间的推移也展现出了稳定的增长,且会达到较高的数值,原因就是接触时间不足1小时的情况下CeO2的还原程度较低。
因此,差异化处理那些依赖于自身化学性质和尺寸而发生快速变化的纳米粒子是可行的,比如Ag0[207]。同样地,有机分子对ENMs表面的修饰至少在较短的时期内增加了粒子对生物聚集体的亲和力。文本框7.3提供了此部分内容的总结性信息。
文本框 7.3 滞留、聚集与沉积模型的使用
绝大多数ENMs最终将存在于干污泥和堆肥中。这些固体污泥有时会作为肥料施用于农业。然而不论是对于纳米粒子进入地表水数据的模型研究[186],还是就纳米材料对植物或根系有机物(如虫类及细菌)的影响方面的实验室研究都为数很少。近期的一篇论文对硫化银在堆肥中的稳定性进行了阐述[210],但应该指出的是实验并没有选取含ENMs的产品来开展相应的研究,而且实验的模拟条件也和现实相去甚远,比如废水的处理需要经过很多道工序,并且会产生含有ENMs的污泥,暂且不论这些纳米粒子是否会发生变化。我们已经知道在污泥处理厂中,一些纳米材料诸如ZnO、Ag0、CeO2、CuO等会发生不同程度的变化,但是TiO2则相对较为稳定。实际上,对于经过处理厂处理的物质的迁移,其在被施用于土壤后的可能变化及其在根系中与植物及细菌的反应,以及向地表水的转移等方面均未有深层次的研究。
全球范围内开展对含ENMs废水生物处理效果的研究团队并不多。在欧洲,主要集中在英国、法国及瑞士。瑞士及法国的团队采用较为相似的手段对其中变化机制的相关课题进行了较深入的研究。
另外美国纳米技术环境应用中心联合法国国家科学中心、法国SERENADE实验室、英国、澳大利亚及其他国家的研究人员开展了“纳米技术与环境——大西洋倡议项目”。该项目由美国资助,致力于对城市污水处理厂中纳米材料的相关变化及其对处理工艺以及植物和土壤有机体的影响等方面进行研究。不过,该项研究并不建议将经污水处理厂处理过的含纳米材料堆肥污泥直接供给下游应用。
由法美联合开展的另一项目旨在评估污水处理厂污泥中纳米材料造成的影响及其可能发生的转移,项目同时涉及:(1)通过采用美国纳米技术环境应用中心的生物群落研究纳米材料向表面水体的扩散及转移;(2)对于人类食用植物的可捕获性之量化分析;(3)污泥的农业应用对土壤根系中菌落的直接及间接影响。
世界范围内的研究团队针对在有氧及厌氧条件下纳米材料对各种菌群的影响进行了研究,特别是厌氧条件,因为这是制备最终材料的重要阶段,尤其在农业应用中。
目前可总结为以下几点。
(1)污水处理厂中发生的化学变化是影响纳米材料研究的重要因素,如氧化还原后产生的溶解能力改变现象。日常商品中存在的纳米粒子的表面功能化可能会减缓这类反应的进行,因为与细菌聚集体的接触受到限制,使得物质原来的氧化或还原状态保持的更加持久。
(2)对不同处理阶段操作的研究依然处于初级阶段,因此根据纳米材料的数量及其表面状况,还需要更加系统的方法去研究菌落在有氧或无氧条件下的变化。一些实验结果包括高浓度时的研究结果似乎并不能完全令人信服。
(3)细菌聚集体对纳米材料滞留能力的预测可以利用分配系数(γ)来表征,其表达了纳米粒子或纳米材料在水相及在固相中以细菌聚集体的形式存在的分配比例。
(4)经污水处理厂处理后的ENMs的迁移,包括其随污泥施用于土壤后可能的变化及其在根系中与植物及细菌发生的反应,向地表水的转移等等这些问题,均未得到深层次的研究。
当前的研究经常涉及到活化污泥反应装置的使用,而对于厌氧条件下的处理环节还未进行全面探究。同样的,当前很多研究围绕非表面功能化纳米粒子,但生活中大量消费类产品中含有的都是表面功能化纳米粒子(如化妆品、塑料制品、农业食品、服装面料及油漆等)。针对含ENMs产品的退化及衰变以及污水处理厂中纳米粒子表面改变方面的研究无人涉及。为弥补这些空白,以下措施似乎看起来非常必要。
(1)建立包含所有相关工艺环节的大型中试处理厂,获取完整处理工艺数据。
(2)研究现实中以同样方式不断生产且广为使用的如化妆品、油漆及农产品等商品使用后的残余物(例如,请参阅欧洲NEPHH计划),确保ENMs被排放到水中(充分稀释)后以及在处理厂中的各个阶段所发生的变化都能够被监测到。已经有研究人员展开了对化妆品表面功能化后NMMs在较为温和的条件下发生的变化[192,217,218,220,221],以及在实验性工厂有氧反应条件下纳米助剂(柠檬酸修饰的CeO2)表面功能化后的变化[201]。但此类相关研究的开展依然有限。
(3)评估污泥农业应用的影响,如建立类似于土壤测试(RHIZO)的测试方法,对金属转移至植物的风险进行评估[222]。这方面的实验需要在与现实环境相似的条件下进行,污泥中纳米材料的浓度也不能很高。对纳米材料的转移过程采用同位素跟踪应该是很有效的方法。三维可视化工具如X射线成像虽未广泛在实验室应用,但其可对在不同组织(植物、活有机体等)中的重元素进行定位。最后,需要结合真实土壤进行研究,因为ENMs的具体表现细节还与土壤中千差万别的成分或构成有很大的关系。
学科交叉是该研究领域的突出特征,要想研究对生物体(植物、细菌等)的影响必然涉及生物多样性及生长方面的知识,同时又需要对变化及转移机制有所了解,而这却是物理化学家和研究多孔介质转移的专家所擅长的领域。
☆Nanomaterials in Waste Streams: Current Knowledge on Risks and Impacts一文由位于巴黎的经济合作与发展组织(OECD)以英文版出版,http://dx.doi.org/10.1787/9789264249752-en. © OECD 2016
中国科学院文献情报中心经OECD授权出版该报告的中文版,© 2016 中国科学院文献情报中心。中国科学院文献情报中心对中文翻译的质量及其与原文的一致性负责,如果出现译文与原文不一致的地方以原文内容为准。
本报告分为两部分,本期刊登下半部分。
翻译:朱海峰 审校:马建华
The authors have declared that no competing interests exist.
| [95] |
|
| [96] |
|
| [97] |
DOI:10.1007/s11051-013-1692-4
URL
[Cite within: 1]
Engineered nanomaterials (ENMs) are now becoming a significant fraction of the material flows in the global economy. We are already reaping the benefits of improved energy efficiency, material use reduction, and better performance in many existing and new applications that have been enabled by these technological advances. As ENMs pervade the global economy, however, it becomes important to understand their environmental implications. As a first step, we combined ENM market information and material flow modeling to produce the first global assessment of the likely ENM emissions to the environment and landfills. The top ten most produced ENMs by mass were analyzed in a dozen major applications. Emissions during the manufacturing, use, and disposal stages were estimated, including intermediate steps through wastewater treatment plants and waste incineration plants. In 2010, silica, titania, alumina, and iron and zinc oxides dominate the ENM market in terms of mass flow through the global economy, used mostly in coatings/paints/pigments, electronics and optics, cosmetics, energy and environmental applications, and as catalysts. We estimate that 63–9102% of over 260,000–309,000 metric tons of global ENM production in 2010 ended up in landfills, with the balance released into soils (8–2802%), water bodies (0.4–702%), and atmosphere (0.1–1.502%). While there are considerable uncertainties in the estimates, the framework for estimating emissions can be easily improved as better data become available. The material flow estimates can be used to quantify emissions at the local level, as inputs for fate and transport models to estimate concentrations in different environmental compartments.
|
| [98] |
DOI:10.1016/j.envint.2010.08.005
PMID:20832119
URL
[Cite within: 2]
Recent exponential growth in the development of nanomaterials (NMs) and nanoproducts is premised on the provision of novel benefits to the society-through the exploitation of their unique industrial and biomedical applications like medical imaging, fabrics in textiles, tissue engineering, nanocomposites, bioremediation, and biomedicine. These NMs and nanoproducts have increased in quantity and volume from few kilograms to thousands of tonnes over the last fifteen to twenty years, and their uncontrolled release into the environment is anticipated to grow dramatically in future. However, their potential impacts to the biological systems are unknown. Among the key present challenges in the waste management sector include the emergence of nanowastes; however, the effectiveness and the capability of the current systems to handle them are yet to be established. Because of limited studies on nanowastes management, in this paper, three-fold objectives are pursued, namely; (i) to raise concerns related to the alarming increases of uncontrolled releases of NMs into the environment through nanowastes, (ii) examine the unique challenges nanowastes pose to the waste management systems-both from technological and legislative perspectives, and (iii) summarize results of the first nanowastes classification formalism in order to elucidate the potential challenges of waste streams containing nanoscale dimension materials to the present waste management paradigm. Finally, the article closes by summarizing several proactive steps of enhancing effective long-term and responsible management of nanowastes.
|
| [99] |
DOI:10.1007/s11051-012-1109-9
URL
Not much is known so far about the amounts of engineered nanomaterials (ENM) that are produced but this information is crucial for environmental exposure assessment. This paper provides worldwide and Europe-wide estimates for the production and use of ten different ENM (TiO 2 , ZnO, FeO x , AlO x , SiO 2 , CeO 2 , Ag, quantum dots, CNT, and fullerenes) based on a survey sent to companies producing and using ENM. The companies were asked about their estimate of the worldwide or regional market and not about their company-specific production, information that they would be less likely to communicate. The study focused on the actual production quantities and not the production capacities. The survey also addressed information on distribution of the produced ENM to different product categories. The results reveal that some ENM are produced in Europe in small amounts (less than 10t/year for Ag, QDs and fullerenes). The most produced ENM is TiO 2 with up to 10,000t of worldwide production. CeO 2 , FeO x , AlO x , ZnO, and CNT are produced between 100 and 1000t/year. The data for SiO 2 cover the whole range from less than 10 to more than 10,000t/year, which is indicative of problems related to the definition of this material (is pyrogenic silica considered an ENM or not?). For seven ENM we have obtained the first estimates for their distribution to different product categories, information that also forms the base for life-cycle based exposure analysis.
|
| [100] |
|
| [101] |
DOI:10.1021/es071818o
PMID:18504950
URL
A large number of applications using manufactured nanoparticles of less than 100 nm are currently being introduced into industrial processes. There is an urgent need to evaluate the risks of these novel particles to ensure their safe production, handling, use, and disposal. However, today we lack even rudimentary knowledge about type and quantity of industrially used manufactured nanoparticles and the level of exposure in Swiss industry. The goal of this study was to evaluate the use of nanoparticles, the currently implemented safety measures, and the number of potentially exposed workers in all types of industry. To evaluate this, a targeted telephone survey was conducted among health and safety representatives from 197 Swiss companies. The survey showed that nanoparticles are already used in many industrial sectors; not only in companies in the new field of nanotechnology, but also in more traditional sectors, such as paints. Forty-three companies declared to use or produce nanoparticles, and 11 imported and traded with prepackaged goods that contain nanoparticles. The following nanoparticles were found to be used in considerable quantities (> 1000 kg/year per company): Ag, Al-Ox, Fe-Ox, SiO2, TiO2, and ZnO. The median reported quantity of handled nanoparticles was 100 kg/year. The production of cosmetics, food, paints, powders, and the treatment of surfaces used the largest quantities of these nanoparticles. Generally, the safety measures were found to be higher in powder-based than in liquid-based applications. However, the respondents had many open questions about best practices, which points to the need for rapid development of guidelines and protection strategies.
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
DOI:10.1016/j.scitotenv.2011.12.030
PMID:22265599
URL
[Cite within: 5]
If nanotechnology proves to be successful for bulk applications, large quantities of nanocomposites are likely to end up in municipal solid waste incineration (MSWI) plants. Various studies indicate that nanoobjects might be harmful to human health and the environment. At this moment there is no evidence that all nanoobjects are safely removed from the off-gas when incinerating nanocomposites in MSWI plants. This paper presents a preliminary assessment of the fate of nanoobjects during waste incineration and the ability of MSWI plants to remove them. It appears that nanoobject emission levels will increase if bulk quantities of nanocomposites end up in municipal solid waste. Many primary and secondary nanoobjects arise from the incineration of nanocomposites and removal seems insufficient for objects that are smaller than 100nm. For the nanoobjects studied in this paper, risks occur for aluminum oxide, calcium carbonate, magnesium hydroxide, POSS, silica, titanium oxide, zinc oxide, zirconia, mica, montmorillonite, talc, cobalt, gold, silver, carbon black and fullerenes. Since this conclusion is based on a desktop study without accompanying experiments, further research is required to reveal which nanoobjects will actually be emitted to the environment and to determine their toxicity to human health.
|
| [106] |
|
| [107] |
DOI:10.1038/nnano.2012.64
PMID:22609690
URL
[Cite within: 2]
More than 100 million tonnes of municipal solid waste are incinerated worldwide every year. However, little is known about the fate of nanomaterials during incineration, even though the presence of engineered nanoparticles in waste is expected to grow. Here, we show that cerium oxide nanoparticles introduced into a full-scale waste incineration plant bind loosely to solid residues from the combustion process and can be efficiently removed from flue gas using current filter technology. The nanoparticles were introduced either directly onto the waste before incineration or into the gas stream exiting the furnace of an incinerator that processes 200,000 tonnes of waste per year. Nanoparticles that attached to the surface of the solid residues did not become a fixed part of the residues and did not demonstrate any physical or chemical changes. Our observations show that although it is possible to incinerate waste without releasing nanoparticles into the atmosphere, the residues to which they bind eventually end up in landfills or recovered raw materials, confirming that there is a clear environmental need to develop degradable nanoparticles.
|
| [108] |
DOI:10.1039/C3EN00080J
URL
[Cite within: 1]
As the use of nanotechnology in consumer products continues to grow, it is inevitable that some nanomaterials will end up in the waste stream and will be incinerated. Through laboratory-scale incineration of paper and plastic wastes containing nanomaterials, we assessed their effect on emissions of particulate matter (PM) and the effect of incineration on the nanomaterials themselves. The presence of nanomaterials did not significantly influence the particle number emission factor. The PM size distribution was not affected except at very high mass loadings (10 wt%) of the nanomaterial, in which case the PM shifted toward smaller sizes; such loadings are not expected to be present in many consumer products. Metal oxide nanomaterials reduced emissions of particle-bound polycyclic aromatic hydrocarbons. Most of the nanomaterials that remained in the bottom ash retained their original size and morphology but formed large aggregates. Only small amounts of the nanomaterials (0.023 180 mg g 1 of nanomaterial) partitioned into PM, and the emission factors of nanomaterials from an incinerator equipped with an electrostatic precipitator are expected to be low. However, a sustainable disposal method for nanomaterials in the bottom ash is needed, as a majority of them partitioned into this fraction and may thus end up in landfills upon disposal of the ash.
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
DOI:10.1007/s11051-012-0720-0
URL
[Cite within: 2]
Understanding environmental impacts of nanomaterials necessitates analyzing the life cycle profile. The initial emphasis of nanomaterial life cycle studies has been on the environmental and health effects of nanoproducts during the production and usage stages. Analyzing the end-of-life (eol) stage of nanomaterials is also critical because significant impacts or benefits for the environment may arise at that particular stage. In this article, the Woodrow Wilson Center's Project on Emerging Nanotechnologies (PEN) Consumer Products Inventory (CPI) model was used, which contains a relatively large and complete nanoproduct list (1,014) as of 2010. The consumer products have wide range of applications, such as clothing, sports goods, personal care products, medicine, as well as contributing to faster cars and planes, more powerful computers and satellites, better micro and nanochips, and long-lasting batteries. In order to understand the eol cycle concept, we allocated 1,014 nanoproducts into the nine end-of-life categories (e.g., recyclability, ingestion, absorption by skin/public sewer, public sewer, burning/landfill, landfill, air release, air release/public sewer, and other) based on probable final destinations of the nanoproducts. This article highlights the results of this preliminary assessment of end-of-life stage of nanoproducts. The largest potential eol fate was found to be recyclability, however little literature appears to have evolved around nanoproduct recycling. At lower frequency is dermal and ingestion human uptake and then landfill. Release to water and air are much lower potential eol fates for current nanoproducts. In addition, an analysis of nano-product categories with the largest number of products listed indicated that clothes, followed by dermal-related products and then sports equipment were the most represented in the PEN CPI (http://www.nanotechproject.org/inventories/consumer/browse/categories/http://www.nanotechproject.org/inventories/consumer/browse/categories/ 2010).
|
| [113] |
|
| [114] |
DOI:10.1007/s11051-014-2394-2
PMID:24944519
URL
[Cite within: 5]
Abstract Information related to the potential environmental exposure of engineered nanomaterials (ENMs) in the solid waste management phase is extremely scarce. In this paper, we define nanowaste as separately collected or collectable waste materials which are or contain ENMs, and we present a five-step framework for the systematic assessment of ENM exposure during nanowaste management. The framework includes deriving EOL nanoproducts and evaluating the physicochemical properties of the nanostructure, matrix properties and nanowaste treatment processes as well as transformation processes and environment releases, eventually leading to a final assessment of potential ENM exposure. The proposed framework was applied to three selected nanoproducts: nanosilver polyester textile, nanoTiO 2 sunscreen lotion and carbon nanotube tennis racquets. We found that the potential global environmental exposure of ENMs associated with these three products was an estimated 0.5-143 Mg/year, which can also be characterised qualitatively as medium, medium, low, respectively. Specific challenges remain and should be subject to further research: (1) analytical techniques for the characterisation of nanowaste and its transformation during waste treatment processes, (2) mechanisms for the release of ENMs, (3) the quantification of nanowaste amounts at the regional scale, (4) a definition of acceptable limit values for exposure to ENMs from nanowaste and (5) the reporting of nanowaste generation data.
|
| [115] |
|
| [116] |
DOI:10.1007/s11051-013-1692-4
URL
[Cite within: 5]
Engineered nanomaterials (ENMs) are now becoming a significant fraction of the material flows in the global economy. We are already reaping the benefits of improved energy efficiency, material use reduction, and better performance in many existing and new applications that have been enabled by these technological advances. As ENMs pervade the global economy, however, it becomes important to understand their environmental implications. As a first step, we combined ENM market information and material flow modeling to produce the first global assessment of the likely ENM emissions to the environment and landfills. The top ten most produced ENMs by mass were analyzed in a dozen major applications. Emissions during the manufacturing, use, and disposal stages were estimated, including intermediate steps through wastewater treatment plants and waste incineration plants. In 2010, silica, titania, alumina, and iron and zinc oxides dominate the ENM market in terms of mass flow through the global economy, used mostly in coatings/paints/pigments, electronics and optics, cosmetics, energy and environmental applications, and as catalysts. We estimate that 63–9102% of over 260,000–309,000 metric tons of global ENM production in 2010 ended up in landfills, with the balance released into soils (8–2802%), water bodies (0.4–702%), and atmosphere (0.1–1.502%). While there are considerable uncertainties in the estimates, the framework for estimating emissions can be easily improved as better data become available. The material flow estimates can be used to quantify emissions at the local level, as inputs for fate and transport models to estimate concentrations in different environmental compartments.
|
| [117] |
DOI:10.1016/j.copbio.2013.11.008
PMID:24863899
URL
[Cite within: 3]
Manufactured nanomaterials (MNMs) are increasingly incorporated into everyday products and thus are entering the environment via manufacturing, product use, and waste disposal. Still, understanding MNM environmental hazards and fates lags MNM industry growth. To catch up, keep pace, and influence future MNM safe design strategies, rapid safety assessments are needed. Bacteria are important ecological nanotoxicology targets to consider when assessing MNM safety: bacteria are exposed to MNMs in water, sewage, soils, and sediments, wherein they influence MNM fates; bacteria can also be impacted ith potential health and ecosystem consequences. Routinely using bacteria for assessing MNMs would promote effective management of the environmental risks of this rapidly growing industry, but appropriate protocols and policies for this assessment need to be instituted.
|
| [118] |
DOI:10.2134/jeq2009.0423
PMID:21284287
URL
[Cite within: 3]
Abstract With the fast development of nanotechnology, engineered nanomaterials (ENMs) will inevitably be introduced into the various environment. Increasing studies showed the toxiccity of various ENMs, which raises concerns over their fate and transport in the environment. This review focuses on advances in the research on environmental transport and fate of ENMs. Aggregation and suspension behaviors of ENMs determining their fate and transport in aqueous environment are discussed, with emphasis on the influencing factors, including natural colloids, natural organic matter, pH, and ionic strength. Studies on the transport of ENMs in porous media and its influencing factors are reviewed, and transformation and organismcleansing, as two fate routes of ENMs in the environment, are addressed. Future research directions and outlook in the environmental transport and fate of ENMs are also presented.
|
| [119] |
DOI:10.1016/j.wasman.2012.03.019
PMID:22608471
Magsci
URL
[Cite within: 7]
Abstract Escalating production and subsequent incorporation of engineered nanomaterials in consumer products increases the likelihood of nanomaterials being discarded in landfills. Although direct measurement of particle disposal has not yet occurred, life cycle assessments suggest that over 50% of nanomaterials produced will eventually reside in landfills. Laboratory-scale experiments were conducted to evaluate how organics (humic acid: 20-800 mg/L), ionic strength (100-400 mM NaCl), and pH (6-8) typical of mature leachates influence carbon nanotube surface charge, relative stability, and mobility through representative solid waste environments. Results from the batch experiments suggest that the presence of high molecular weight organics, such as humic acid, acts to stabilize carbon nanotubes present in leachate, even at high ionic strengths (>100 mM NaCl). These results also suggest that in mature landfill leachate, as long as humic acid is present, ionic strength (when represented as NaCl) will be a dominant factor influencing nanomaterial stability. Column experiment results indicate the carbon nanotubes may be mobile through solid waste, suggesting particle placement within landfills needs to be examined more closely. Copyright 2012 Elsevier Ltd. All rights reserved.
|
| [120] |
DOI:10.1021/es7029637
PMID:18605569
URL
[Cite within: 2]
The aim of this study was to use a life-cycle perspective to model the quantities of engineered nanoparticles released into the environment. Three types of nanoparticles were studied: nano silver (nano-Ag), nano TiO2 (nano-TiO2), and carbon nanotubes (CNT). The quantification was based on a substance flow analysis from products to air, soil, and water in Switzerland. The following parameters were used as model inputs: estimated worldwide production volume, allocation of the production volume to product categories, particle release from products, and flow coefficients within the environmental compartments. The predicted environmental concentrations (PEC) were then compared to the predicted no effect concentrations (PNEC) derived from the literature to estimate a possible risk. The expected concentrations of the three nanoparticles in the different environmental compartments vary widely, caused by the different life cycles of the nanoparticle-containing products. The PEC values for nano-TiO2 in water are 0.7--16 microg/L and close to or higher than the PNEC value for nano-TiO2 (< 1 microg/L). The risk quotients (PEC/PNEC) for CNT and nano-Ag were much smaller than one, therefore comprising no reason to expect adverse effects from those particles. The results of this study make it possible for the first time to carry out a quantitative risk assessment of nanoparticles in the environment and suggest further detailed studies of nano-TiO2.
|
| [121] |
DOI:10.1177/0960327110384525
PMID:20921061
URL
[Cite within: 1]
The burgeoning nanotechnology industry is rapidly generating new forms of waste streams generically referred herein as nanowastes. However, little is known about the fate and behavior of these waste streams and their impacts thereof in different ecological systems despite their increasingly widespread dispersion into the environment through production, distribution, handling, and nanomaterials (NMs) incorporation into bulk products processes. In this paper, risk assessment of nanotechnology from a waste management perspective was examined to elucidate potential new forms of challenges nanowastes may likely pose to the current legislative and waste management systems. This was through the identification of several knowledge gaps that merit urgent attention in order to increase our collective understanding of managing nanowastes safely, responsibly, and sustainably. The paper presents the identified gaps and consequently proposes a qualitative risk assessment of nanowastes to address some of the current challenges. The applicability of the proposed model is illustrated through several examples. In addition, the first nanowastes classification protocol presented in this article show that a given nanomaterial may result in generating nanowaste streams of different forms with variant hazard levels ranging from benign to extremely being hazardous waste streams - a dramatic phenomenon from the conventional waste streams due to macroscale chemicals. The study shows that it is in the early days to draw broad generic classification of different nanowastes, and each stream may require their risk profile be assessed on a case-by-case basis. We conclude by presenting several recommendations on what needs to be done in dealing with nanowastes as means of avoiding unintended long-term consequences of nanotechnology.
|
| [122] |
DOI:10.1016/j.envint.2013.04.003
PMID:23708563
URL
[Cite within: 5]
It can be concluded that in general, significant release of CNTs from products and articles is unlikely except in manufacturing and subsequent processing, tires, recycling, and potentially in textiles. However except for high energy machining processes, most likely the resulting exposure for these scenarios will be low and to a non-pristine form of CNTs. Actual exposure studies, which quantify the amount of material released should be conducted to provide further evidence for this conclusion.
|
| [123] |
DOI:10.1016/j.wasman.2012.01.009
PMID:22317796
URL
[Cite within: 3]
Abstract Silver nanoparticles (AgNPs, nanosilver) released from industrial activities and consumer products may be disposed directly or indirectly in sanitary landfills. To determine the impact of AgNPs on anaerobic digestion of landfill waste, municipal solid waste (MSW) was loaded in identical landfill bioreactors (9L volume each) and exposed to AgNPs (average particle size=21nm) at the final concentrations of 0, 1, and 10mgAg/kg solids. The landfill anaerobic digestion was carried out for more than 250 days, during which time the cumulative biogas production was recorded automatically and the chemical property changes of leachates were analyzed. There were no significant differences in the cumulative biogas volume or gas production rate between the groups of control and 1mgAg/kg. However, landfill solids exposed to AgNPs at 10mg/kg resulted in the reduced biogas production, the accumulation of volatile fatty acids (including acetic acid), and the prolonged period of low leachate pH (between 5 and 6). Quantitative PCR results after day 100 indicated that the total copy numbers of 16S rRNA gene of methanogens in the groups of control and 1mgAgNPs/kg were 1.97±0.21×10(7) and 0.90±0.03×10(7), respectively. These numbers were significantly reduced to 5.79±2.83×10(5)(copies/mL) in the bioreactor treated with 10mgAgNPs/kg. The results suggest that AgNPs at the concentration of 1mg/kg solids have minimal impact on landfill anaerobic digestion, but a concentration at 10mg/kg or higher inhibit methanogenesis and biogas production from MSW. Copyright 08 2012 Elsevier Ltd. All rights reserved.
|
| [124] |
DOI:10.1016/j.jhazmat.2005.08.010
PMID:16314043
URL
[Cite within: 1]
In this paper, the technical applicability and treatment performance of physico-chemical techniques (individual and/or combined) for landfill leachate are reviewed. A particular focus is given to coagulation–flocculation, chemical precipitation, ammonium stripping, membrane filtration and adsorption. The advantages and limitations of various techniques are evaluated. Their operating conditions such as pH, dose required, characteristics of leachate in terms of chemical oxygen demand (COD) and NH 3 –N concentration and treatment efficiency are compared. It is evident from the survey of 118 papers (1983–2005) that none of the individual physico-chemical techniques is universally applicable or highly effective for the removal of recalcitrant compounds from stabilized leachate. Among the treatments reviewed in this article, adsorption, membrane filtration and chemical precipitation are the most frequently applied and studied worldwide. Both activated carbon adsorption and nanofiltration are effective for over 95% COD removal with COD concentrations ranging from 5690 to 17,00002mg/L. About 98% removal of NH 3 –N with an initial concentration ranging from 3260 to 561802mg/L has been achieved using struvite precipitation. A combination of physico-chemical and biological treatments has demonstrated its effectiveness for the treatment of stabilized leachate. Almost complete removal of COD and NH 3 –N has been accomplished by a combination of reverse osmosis (RO) and an upflow anaerobic sludge blanket (UASB) with an initial COD concentration of 35,00002mg/L and NH 3 –N concentration of 160002mg/L and/or RO and activated sludge with an initial COD concentration of 644002mg/L and NH 3 –N concentration of 115302mg/L. It is important to note that the selection of the most suitable treatment method for landfill leachate depends on the characteristics of landfill leachate, technical applicability and constraints, effluent discharge alternatives, cost-effectiveness, regulatory requirements and environmental impact.
|
| [125] |
|
| [126] |
DOI:10.1021/ac303636s
PMID:23427995
Magsci
URL
[Cite within: 2]
While nanoparticles occur naturally in the environment and have been intentionally used for centuries, the production and use of engineered nanoparticles has seen a recent spike, which makes environmental release almost certain. Therefore, recent efforts to characterize the toxicity of engineered nanoparticles have focused on the environmental implications, including exploration of toxicity to organisms from wide-ranging parts of the ecosystem food webs. Herein, we summarize the current understanding of toxicity of engineered nanoparticles to representatives of various trophic levels, including bacteria, plants, and multicellular aquatic/terrestrial organisms, to highlight important challenges within the field of econanotoxicity, challenges that analytical chemists are expertly poised to address.
|
| [127] |
DOI:10.1016/j.wasman.2013.06.022
PMID:23871188
URL
[Cite within: 2]
The presence in waste of emerging pollutants (EPs), whose behaviours and effects are not well understood, may present unexpected health and environmental risks and risks for the treatment processes themselves. EP may include substances that are newly detected in the environment, substances already identified as risky and whose use in items is prohibited (but which may be present in old or imported product waste) or substances already known but whose recent use in products can cause problems during their future treatment as waste. Several scientific studies have been conducted to assess the presence of EP in waste, but they are mostly dedicated to a single category of substance or one particular waste treatment. In the absence of a comprehensive review focused on the impact of the presence of EP on waste treatment schemes, the authors present a review of the key issues associated with the treatment of waste containing emerging pollutants. This review presents the typologies of emerging pollutants that are potentially present in waste along with the major challenges for each treatment scheme (recycling, composting, digestion, incineration, landfilling and wastewater treatment). All conventional treatment processes are affected by these new pollutants, and they were almost never originally designed to consider these substances. In addition to these general aspects, a comprehensive review of available data, projects and future R&D needs related to the impact of nanoparticles on waste treatment is presented as a case study.
|
| [128] |
|
| [129] |
DOI:10.1016/j.wasman.2013.04.014
PMID:23746986
URL
[Cite within: 1]
The potential colloids release from a large panel of 25 solid industrial and municipal waste leachates, contaminated soil, contaminated sediments and landfill leachates was studied. Standardized leaching, cascade filtrations and measurement of element concentrations in the microfiltrate (MF) and ultrafiltrate (UF) fraction were used to easily detect colloids potentially released by waste. Precautions against CO(2) capture by alkaline leachates, or bacterial re-growth in leachates from wastes containing organic matter should be taken. Most of the colloidal particles were visible by transmission electron microscopy with energy dispersion spectrometry (TEM-EDS) if their elemental MF concentration is greater than 200 gl(-1). If the samples are dried during the preparation for microscopy, neoformation of particles can occur from the soluble part of the element. Size distribution analysis measured by photon correlation spectroscopy (PCS) were frequently unvalid, particularly due to polydispersity and/or too low concentrations in the leachates. A low sensitivity device is required, and further improvement is desirable in that field. For some waste leachates, particles had a zeta potential strong enough to remain in suspension. Mn, As, Co, Pb, Sn, Zn had always a colloidal form (MF concentration/UF concentration>1.5) and total organic carbon (TOC), Fe, P, Ba, Cr, Cu, Ni are partly colloidal for more than half of the samples). Nearly all the micro-pollutants (As, Ba, Co, Cr, Cu, Mo, Ni, Pb, Sb, Sn, V and Zn) were found at least once in colloidal form greater than 100 gl(-1). In particular, the colloidal forms of Zn were always by far more concentrated than its dissolved form. The TEM-EDS method showed various particles, including manufactured nanoparticles (organic polymer, TiO(2), particles with Sr, La, Ce, Nd). All the waste had at least one element detected as colloidal. The solid waste leachates contained significant amount of colloids different in elemental composition from natural ones. The majority of the elements were in colloidal form for wastes of packaging (3), a steel slag, a sludge from hydrometallurgy, composts (2), a dredged sediment (#18), an As contaminated soil and two active landfill leachates. These results showed that cascade filtration and ICP elemental analysis seems valid methods in this field, and that electronic microscopy with elemental detection allows to identify particles. Particles can be formed from dissolved elements during TEM sample preparation and cross-checking with MF and UF composition by ICP is useful. The colloidal fraction of leachate of waste seems to be a significant source term, and should be taken into account in studies of emission and transfer of contaminants in the environment. Standardized cross-filtration method could be amended for the presence of colloids in waste leachates.
|
| [130] |
|
| [131] |
|
| [132] |
DOI:10.1016/j.envint.2010.08.005
PMID:20832119
Magsci
URL
[Cite within: 9]
Recent exponential growth in the development of nanomaterials (NMs) and nanoproducts is premised on the provision of novel benefits to the society-through the exploitation of their unique industrial and biomedical applications like medical imaging, fabrics in textiles, tissue engineering, nanocomposites, bioremediation, and biomedicine. These NMs and nanoproducts have increased in quantity and volume from few kilograms to thousands of tonnes over the last fifteen to twenty years, and their uncontrolled release into the environment is anticipated to grow dramatically in future. However, their potential impacts to the biological systems are unknown. Among the key present challenges in the waste management sector include the emergence of nanowastes; however, the effectiveness and the capability of the current systems to handle them are yet to be established. Because of limited studies on nanowastes management, in this paper, three-fold objectives are pursued, namely; (i) to raise concerns related to the alarming increases of uncontrolled releases of NMs into the environment through nanowastes, (ii) examine the unique challenges nanowastes pose to the waste management systems-both from technological and legislative perspectives, and (iii) summarize results of the first nanowastes classification formalism in order to elucidate the potential challenges of waste streams containing nanoscale dimension materials to the present waste management paradigm. Finally, the article closes by summarizing several proactive steps of enhancing effective long-term and responsible management of nanowastes.
|
| [133] |
|
| [134] |
|
| [135] |
DOI:10.1016/j.wasman.2010.08.004
PMID:20797842
Magsci
URL
[Cite within: 5]
Reinhart DR, Berge ND, Santra S, Bolyard SC.
|
| [136] |
DOI:10.1016/j.crte.2014.10.004
URL
[Cite within: 4]
Interdisciplinarity is of first importance to evaluate the risks associated with nanotechnology. The reasons are that nanomaterials are very new materials that combine nano-sizes and new reactivities. The complexity comes from the very low concentrations of nanomaterials in the environmental medium, the transformations of the nanomaterials due to the reactivity of the surface, the transfer in the environmental media, particularly in the presence of liquid water (soils, sediments, surface water), which implies an association with natural colloids (organic or minerals) and blockage in some compartments. These properties govern the hazard that strongly depends on exposure and speciation.
|
| [137] |
|
| [138] |
|
| [139] |
DOI:10.1038/nnano.2012.64
PMID:22609690
URL
[Cite within: 1]
More than 100 million tonnes of municipal solid waste are incinerated worldwide every year. However, little is known about the fate of nanomaterials during incineration, even though the presence of engineered nanoparticles in waste is expected to grow. Here, we show that cerium oxide nanoparticles introduced into a full-scale waste incineration plant bind loosely to solid residues from the combustion process and can be efficiently removed from flue gas using current filter technology. The nanoparticles were introduced either directly onto the waste before incineration or into the gas stream exiting the furnace of an incinerator that processes 200,000 tonnes of waste per year. Nanoparticles that attached to the surface of the solid residues did not become a fixed part of the residues and did not demonstrate any physical or chemical changes. Our observations show that although it is possible to incinerate waste without releasing nanoparticles into the atmosphere, the residues to which they bind eventually end up in landfills or recovered raw materials, confirming that there is a clear environmental need to develop degradable nanoparticles.
|
| [140] |
DOI:10.1897/07-282.1
PMID:18333679
URL
[Cite within: 3]
Emerging nanotechnologies hold great promise for creating new means of detecting pollutants, cleaning polluted waste streams, and recovering materials before they become wastes, thereby protecting environmental quality. Studies focusing on the different advantages of nanoscience and nanotechnology abound in the literature, but less research effort seems to be directed toward studying the fate and potential impacts of wastes that will be generated by this technology. Using a combination of biogeochemical and toxicological methods, we conducted a preliminary investigation of the potential environmental fate of Hg as an example pollutant bound to nanomaterials used in treatment of gas effluents. Methylation of Hg sorbed onto SiO(2)-TiO(2) nanocomposites was used as a proxy for Hg bioavailability to sedimentary microorganisms, and the FluoroMetPLATE assay was used to assess the toxicity of both virgin and Hg-loaded SiO(2)-TiO(2) nanocomposites. Our results show that the bioavailability of Hg sorbed onto SiO(2)-TiO(2) nanocomposites to sedimentary microorganisms is pH dependent, with decreasing reaction rates as the pH increases from 4 to 6. Toxicity tests conducted using liquid extracts obtained by leaching of Hg-loaded SiO(2)-TiO(2) nanocomposites with the synthetic precipitation leaching procedure solution showed an average inhibition of 84% (vs 57% for virgin SiO(2)-TiO(2) nanocomposites). These results suggest that Hg sorbed onto engineered nanoparticles could become bioavailable and toxic if introduced into natural systems. Accordingly, studies focusing on the environmental implications of nanomaterials should include determination of the fate and impacts of pollutants that enter the environment bound to engineered nanomaterials.
|
| [141] |
|
| [142] |
|
| [143] |
DOI:10.1021/es060407p
PMID:17180993
URL
[Cite within: 2]
Single-walled carbon nanotubes (SWNT) are finding increasing use in consumer electronics and structural composites. These nanomaterials and their manufacturing byproducts may eventually reach estuarine systems through wastewater discharge. The acute and chronic toxicity of SWNTs were evaluated using full life-cycle bioassays with the estuarine copepod Amphiascus tenuiremis (ASTM method E-2317-04). A synchronous cohort of naupliar larvae was assayed by culturing individual larvae to adulthood in individual 96-well microplate wells amended with SWNTs in seawater. Copepods were exposed to “as prepared” (AP) SWNTs, electrophoretically purified SWNTs, or a fluorescent fraction of nanocarbon synthetic byproducts. Copepods ingesting purified SWNTs showed no significant effects on mortality, development, and reproduction across exposures (p<0.05). In contrast, exposure to the more complex AP-SWNT mixture significantly increased life-cycle mortality, reduced fertilization rates, and reduced molting success in the ...
|
| [144] |
DOI:10.1016/j.envpol.2011.03.003
Magsci
[Cite within: 1]
This work investigates the physical-chemical evolution during artificial aging in water of four commercialized sunscreens containing TiO2-based nanocomposites. Sunscreens were analyzed in terms of mineralogy and TiO2 concentration. The residues formed after aging were characterized in size, shape, chemistry and surface properties. The results showed that a significant fraction of nano-TiO2 residues was released from all sunscreens, despite their heterogeneous behaviors. A stable dispersion of submicronic aggregates of nanoparticles was generated, representing up to 38 w/w% of the amount of sunscreen, and containing up to 30% of the total nano-TiO2 initially present in the creams. The stability of the dispersion was tested as a function of salt concentration, revealing that in seawater conditions, a major part of these nano-TiO2 residues will aggregate and sediment. These results were put in perspective with consumption and life cycle of sunscreens to estimate the amount of nano-TiO2 potentially released into AQUATIC environment. (C) 2011 Elsevier Ltd. All rights reserved.
|
| [145] |
DOI:10.1021/am301031g
PMID:22853320
URL
[Cite within: 1]
The surfaces of Zn-doped biomagnetite nanostructured particles were functionalized with (3-mercaptopropyl)trimethoxysilane (MPTMS) and used as a high-capacity and collectable adsorbent for the removal of Hg(II) from water. Fourier transform infrared spectroscopy (FTIR) confirmed the attachment of MPTMS on the particle surface. The crystallite size of the Zn-doped biomagnetite was 6517 nm, and the thickness of the MPTMS coating was 655 nm. Scanning transmission electron microscopy and dynamic light scattering analyses revealed that the particles formed aggregates in aqueous solution with an average hydrodynamic size of 826 ± 32 nm. Elemental analyses indicate that the chemical composition of the biomagnetite is Zn(0.46)Fe(2.54)O(4), and the loading of sulfur is 3.6 mmol/g. The MPTMS-modified biomagnetite has a calculated saturation magnetization of 37.9 emu/g and can be separated from water within a minute using a magnet. Sorption of Hg(II) to the nanostructured particles was much faster than other commercial sorbents, and the Hg(II) sorption isotherm in an industrial wastewater follows the Langmuir model with a maximum capacity of 65416 mg/g, indicating two -SH groups bonded to one Hg. This new Hg(II) sorbent was stable in a range of solutions, from contaminated water to 0.5 M acid solutions, with low leaching of Fe, Zn, Si, and S (<10%).
|
| [146] |
DOI:10.1021/je030247m
URL
[Cite within: 1]
The main purpose of this study was to characterize the adsorption and desorption interactions of naphthalene, a model environmental organic pollutant, with C60 fullerene. C60 fullerene was used as a model adsorbent for carbonaceous nanoparticles. Typical batch reactors were used to perform adsorption and desorption experiments. Adsorption and desorption of naphthalene to and from C60 fullerene solids in different aggregation forms was studied, where C60 was used as purchased, deposited as a thin film, or dispersed in water by magnetic mixing. Adsorption and desorption of naphthalene to activated carbon, a common sorbent, was also studied and compared with that of C60. It was found in this study that the enhanced dispersal of C60 could affect the adsorption of naphthalene by several orders of magnitude. A solid-water distribution coefficient of 102.4 mL g-1 was obtained for adsorption of naphthalene to poorly dispersed C60, whereas (104.2 to 104.3) ml g-1 coefficients were obtained for well-dispersed C60 s...
|
| [147] |
DOI:10.1021/es052208w
PMID:16570608
URL
[Cite within: 1]
Abstract Carbon nanomaterials are novel manufactured materials, having widespread potential applications. Adsorption of hydrophobic organic compounds (HOCs) by carbon nanomaterials may enhance their toxicity and affect the fate, transformation, and transport of HOCs in the environment. In this research, adsorption of naphthalene, phenanthrene, and pyrene onto six carbon nanomaterials, including fullerenes, single-walled carbon nanotubes, and multiwalled carbon nanotubes was investigated, which is the first systematic study on polycyclic aromatic hydrocarbons (PAHs) sorption by various carbon nanomaterials. All adsorption isotherms were nonlinear and were fitted well by the Polanyi-Manes model (PMM). Through both isotherm modeling and constructing "characteristic curve", Polanyi theory was useful to describe the adsorption process of PAHs by the carbon nanomaterials. The three fitted parameters (Q0, a, and b) of PMM depended on both PAH properties and the nature of carbon nanomaterials. For different PAHs, adsorption seems to relate with their molecular size, i.e., the larger the molecular size, the lower the adsorbed volume capacity (Q0), but higher a and b values. For different carbon nanomaterials, adsorption seems to relate with their surface area, micropore volume, and the volume ratios of mesopore to micropore. Quantitative relationships between these sorbent properties and the estimated parameters of PMM were obtained. These relationships may represent a first fundamental step toward establishing empirical equations for quantitative prediction of PAH adsorption by carbon nanomaterials and possibly other forms of carbonaceous (geo-) sorbents, and for evaluating their environmental impact. In addition, high adsorption capacity of PAHs by carbon nanotubes may add to their high environmental risks once released to the environment, and result in potential alteration of PAHs fate and bioavailability in the environment.
|
| [148] |
DOI:10.1016/j.aquatox.2007.11.019
PMID:18190976
URL
[Cite within: 1]
The potential of C 60 -nanoparticles (Buckminster fullerenes) as contaminant carriers in aqueous systems was studied in a series of toxicity tests with algae ( Pseudokirchneriella subcapitata ) and crustaceans ( Daphnia magna ). Four common environmental contaminants (atrazine, methyl parathion, pentachlorophenol (PCP), and phenanthrene) were used as model compounds, representing different physico-chemical properties and toxic modes of action. The aggregates of nano-C 60 formed over 2 months of stirring in water were mixed with model compounds 5 days prior to testing. Uptake and excretion of phenanthrene in 4-days-old D. magna was studied with and without addition of C 60 in aqueous suspensions. It was found that 85% of the added phenanthrene sorbed to C 60 -aggregates >200 nm whereas about 10% sorption was found for atrazine, methyl parathion, and pentachlorophenol. In algal tests, the presence of C 60 -aggregates increased the toxicity of phenanthrene with 60% and decreased toxicity of PCP about 1.9 times. Addition of C 60 -aggregates reduced the toxicity of PCP with 25% in tests with D. magna , whereas a more than 10 times increase in toxicity was observed for phenanthrene when results were expressed as water phase concentrations. Thus, results from both toxicity tests show that phenanthrene sorbed to C 60 -aggregates is available for the organisms. For atrazine and methyl parathion no statistically significant differences in toxicities could be observed in algal and daphnid tests as a result of the presence of C 60 -aggregates. In bioaccumulation studies with phenanthrene in D. magna it was found that the uptake of phenanthrene was faster when C 60 was present in suspension and that a 1.7 times higher steady-state concentration was reached in the animals. However, a very fast clearance took place when animals were transferred to clean water resulting in no accumulation of phenanthrene. This study is the first to demonstrate the influence of C 60 -aggregates on aquatic toxicity and bioaccumulation of other environmentally relevant contaminants. The data provided underline that not only the inherent toxicity of manufactured nanoparticles, but also interactions with other compounds and characterisation of nanoparticles in aqueous suspension are of importance for risk assessment of nanomaterials.
|
| [149] |
|
| [150] |
DOI:10.1021/es0352303
PMID:15506213
URL
[Cite within: 1]
The production of significant quantities of engineered nanomaterials will inevitably result in the introduction of these materials to the environment. Mobility in a well-defined porous medium was evaluated for eight particulate products of nanochemistry to assess their potential for migration in porous media such as groundwater aquifers and water treatment plant filters. Contrary to the assertion that nanomaterials present monolithic environmental risks, here we show that these nanomaterials exhibit widely differing transport behaviors. Fullerene-based nanomaterials that had been functionalized to facilitate dispersal in water displayed the highest mobilities, with a calculated potential to migrate approximately 10 m in unfractured sand aquifers. Colloidal aggregates of C60, which have been the focus of recent toxicity studies, were among the least mobile of the nanomaterials evaluated.
|
| [151] |
DOI:10.1016/j.ecoenv.2013.05.022
PMID:23769127
URL
[Cite within: 2]
The use of engineered nanoparticles (ENPs) in a wide range of products is associated with an increased concern for environmental safety due to their potential toxicological and adverse effects. ENPs exert antimicrobial properties through different mechanisms such as the formation of reactive oxygen species, disruption of physiological and metabolic processes. Although there are little empirical evidences on environmental fate and transport of ENPs, biosolids in wastewater most likely would be a sink for ENPs. However, there are still many uncertainties in relation to ENPs impact on the biological processes during wastewater treatment. This review provides an overview of the available data on the plausible effects of ENPs on AS and AD processes, two key biologically relevant environments for understanding ENPs icrobial interactions. It indicates that the impact of ENPs is not fully understood and few evidences suggest that ENPs could augment microbial-mediated processes such as AS and AD. Further to this, wastewater components can enhance or attenuate ENPs effects. Meanwhile it is still difficult to determine effective doses and establish toxicological guidelines, which is in part due to variable wastewater composition and inadequacy of current analytical procedures. Challenges associated with toxicity evaluation and data interpretation highlight areas in need for further research studies.
|
| [152] |
DOI:10.1016/j.powtec.2013.08.025
URL
[Cite within: 5]
The increasing use of nanoparticles will inevitably result in their release into the aquatic environment and thereby cause the exposure of living organisms. Due to their larger surface area, high ratio of particle number to mass, enhanced chemical reactivity, and potential for easier penetration of cells, nanoparticles may be more toxic than larger particles of the same substance. Some researchers have been showing some relations between nanoparticles and certain diseases. However, the doses, surface shapes, material toxicity and persistence of nanoparticles may all be factors in determining harmful biological effects. In order to better evaluate their risks, potential exposure route of nanoparticles has to be taken into consideration as well. Finally, a brief summary of techniques for nanoparticle removal in waters and wastewaters is presented, but it seems that no treatment can absolutely protect the public from exposure to a large-scale dissemination of nanomaterials.
|
| [153] |
|
| [154] |
DOI:10.1021/es302493s
PMID:22920588
URL
[Cite within: 1]
This study investigates the issue of nanoparticles/pollutants cocontamination. By combining viability assays, physicochemical and structural analysis (to probe the As speciation and valence), we assessed how gamma Fe2O3 nanoparticles can affect the cytotoxicity, the intra- and extracellular speciation of As(III). Human dermal fibroblasts were contaminated with gamma Fe2O3 nanoparticles and As(III) considering two scenarios: (i) a simultaneous coinjection of the nanoparticles and As, and (ii) an injection of the I: nanoparticles after 24 h of As adsorption in water. In both scenarios, we did not notice significant changes on the nanoparticles surface charge (zeta potential similar to -10 mV) nor hydrodynamic diameters (similar to 9.50 nm) after 24 h. We demonstrated that the coinjection of gamma Fe2O3 nanoparticles and As in the cellular media strongly affects the complexation of the intracellular As with thiol groups. This significantly increases at low doses the cytotoxicity of the As nonadsorbed at the surface of the nanoparticles. However, once As is adsorbed at the surface the desorption is very weak in the culture medium. This fraction of As strongly adsorbed at the surface is significantly less cytotoxic than As itself. On the basis of our data and the thermodynamics, we demonstrated that any disturbance of the biotransformation mechanisms by the nanoparticles (i.e., surface complexation of thiol groups with the iron atoms) is likely to be responsible for the increase of the As adverse effects at low doses.
|
| [155] |
URL
[Cite within: 1]
is not always obvious and views on how to regulate nanomaterials vary substantially, rangingfrom a ''laissez-faire'' attitude to a Helmut Kaiser Consultancy (cited in Boxall et al. ofnanotechnologies in 2006 to be around $410 million and that these will reach $5.8 billion in 2012.
|
| [156] |
|
| [157] |
DOI:10.1021/es1041892
PMID:21466186
URL
[Cite within: 3]
We investigated the behavior of metallic silver nanoparticles (Ag-NP) in a pilot wastewater treatment plant (WWTP) fed with municipal wastewater. The treatment plant consisted of a nonaerated and an aerated tank and a secondary clarifier. The average hydraulic retention time including the secondary clarifier was 1 day and the sludge age was 14 days. Ag-NP were spiked into the nonaerated tank and samples were collected from the aerated tank and from the effluent. Ag concentrations determined by inductively coupled plasma-mass spectrometry (ICP-MS) were in good agreement with predictions based on mass balance considerations. Transmission electron microscopy (TEM) analyses confirmed that nanoscale Ag particles were sorbed to wastewater biosolids, both in the sludge and in the effluent. Freely dispersed nanoscale Ag particles were only observed in the effluent during the initial pulse spike. X-ray absorption spectroscopy (XAS) measurements indicated that most Ag in the sludge and in the effluent was present as Ag(2)S. Results from batch experiments suggested that Ag-NP transformation to Ag(2)S occured in the nonaerated tank within less than 2 h. Physical and chemical transformations of Ag-NP in WWTPs control the fate, the transport and also the toxicity and the bioavailability of Ag-NP and therefore must be considered in future risk assessments.
|
| [158] |
DOI:10.1016/j.envpol.2010.02.012
PMID:20346555
URL
[Cite within: 2]
Aging in water of a TiO(2)-based nanocomposite used in sunscreen cosmetics has been studied as a function of light and time. It consisted initially in a TiO(2) core, coated with Al(OH)(3) and polydimethylsiloxane (PDMS) layers. Size measurement, coating alteration, and surface charge were followed by laser diffraction, TEM/EDS, ICP-AES and electrophoretic mobility measurement. The nanocomposite rapidly underwent progressive dispersion in the aqueous phase, enabled by the dissolution of the PDMS layer. A stable suspension of colloidal byproducts from 50 to 700nm in size was formed. Their positively charged Al(OH)(3) surface was evidenced with an isoelectric point around 7-8, controlling the dispersion stability. The critical coagulation concentrations measured with NaCl and CaCl(2) was 2 10(-2) and 8 10(-3)M respectively. The presence of natural organic matter affected the colloidal stability according to the NOM/byproduct ratio. A 2 wt% ratio favored bridging flocculation, whereas a 20 wt% ratio induced sterical stabilization.
|
| [159] |
URL
[Cite within: 1]
The integrity of groundwater sources is constantly threatened by contaminant plumes generated by accidental gasoline leakages and leachates escaping landfills. These plumes are of concern due to their proven toxicity to living organisms. Aromatic and chlorinated hydrocarbons, volatile fatty acids, phenols, and ammonia have been found in these leachates. In addition, benzene, toluene, and xylenes (BTX) are major components of gasoline. The lack of oxygen in groundwater makes anaerobic bioremediation desired for the treatment of groundwater contaminated with BTX and chlorinated solvents. With the objective of finding microorganisms capable of BTX and cis-dichloroethylene (cis-DCE) degradation under anaerobic conditions for their use in permeable reactive barriers, different inocula were tested in batch experiments. Toluene was rapidly degraded by several inocula in the presence of alternative electron acceptors. Benzene and m-xylene were eliminated by few of the inocula tested after incubation periods ranging from 244 to 716 days. cis-DCE was highly recalcitrant as no degradation was observed over 440 days. Biological processes have been successfully applied for the treatment of landfill leachates as well. In an effort to provide an effective and economical alternative, an anaerobic-aerobic system was evaluated using a synthetic media simulating the organic and ammonia content of real leachates. The removal of the organic content reached 98% in an upflow anaerobic sludge blanket reactor, and resulted in the formation of methane. During the aerobic process, in an innovative down-flow sponge reactor, ammonia was highly transformed to nitrite and nitrate. Complete nitrification was eventually achieved.The capacity of current wastewater treatment plants for removing nanoparticles has been questioned during the last years. Nanoparticles have been incorporated into numerous applications and their presence in wastewater seems to be inevitable. A laboratory-scale secondary treatment system was set-in to study the behavior of cerium and aluminum oxide nanoparticles during wastewater treatment. The nanoparticles were highly removed, suggesting that secondary treatment is suitable for their elimination. The removal of these nanoparticles was influenced by the pH and organic content of the wastewater. Aluminum nanoparticles proved to be toxic; however the performance of the system for eliminating the organic content was recovered over time.
|
| [160] |
|
| [161] |
DOI:10.1021/es702916h
PMID:18605564
URL
[Cite within: 1]
Abstract The effect of natural organic matter (NOM) characteristics and water quality parameters on NOM adsorption to multiwalled carbon nanotubes (MWNT) was investigated. Isotherm experiment results were fitted well with a modified Freundlich isotherm model that took into account the heterogeneous nature of NOM. The preferential adsorption of the higher molecular weight fraction of NOM was observed by size exclusion chromatographic analysis. Experiments performed with various NOM samples suggested that the degree of NOM adsorption varied greatly depending on the type of NOM and was proportional to the aromatic carbon content of NOM. The NOM adsorption to MWNT was also dependent on water quality parameters: adsorption increased as pH decreased and ionic strength increased. As a result of NOM adsorption to MWNT, a fraction of MWNT formed a stable suspension in water and the concentration of MWNT suspension depended on the amount of NOM adsorbed per unit mass of MWNT. The amount of MWNT suspended in water was also affected by ionic strength and pH. The findings in this study suggested that the fate and transport of MWNT in natural systems would be largely influenced by NOM characteristics and water quality parameters.
|
| [162] |
DOI:10.1021/es903059t
PMID:20184360
URL
[Cite within: 1]
The initial aggregation kinetics of single-walled carbon nanotubes (SWNTs) were studied using time-resolved dynamic light scattering. Aggregation of SWNTs was evaluated in the presence of natural organic matter [Suwannee River humic acid (SRHA)], polysaccharide (alginate), protein [bovine serum albumin (BSA)], and cell culture medium [Luria-Bertani (LB) broth] with varying solution concentrations of monovalent (NaCl) and divalent (CaCl(2)) salts. Increasing salt concentration and adding divalent calcium ions induced SWNT aggregation by screening electrostatic charge and thereby suppressing electrostatic repulsion, similar to observations with aquatic colloidal particles. The presence of biomacromolecules significantly retarded the SWNT aggregation rate. BSA protein molecules were most effective in reducing the rate of aggregation followed by SRHA, LB, and alginate. The slowing of the SWNT aggregation rate in the presence of the biomacromolecules and SRHA can be attributed to steric repulsion originating from the adsorbed macromolecular layer. The remarkably enhanced SWNT stability in the presence of BSA, compared to that with the other biomacromolecules and SRHA, is ascribed to the BSA globular molecular structure that enhances steric repulsion. The results have direct implications for the fate and behavior of SWNTs in aquatic environments and biological media.
|
| [163] |
DOI:10.1021/es800329c
PMID:18767645
URL
[Cite within: 2]
Dissolved organic matter (DOM) has been reported to stabilize () suspensions, which increases concern over the subsequent and of . However, it is unknown exactly which compounds or functional groups cause the stabilization of in natural environments. Naturally occurring tannic acid (TA), which has a large number of aromatic functional groups, was used as a surrogate of DOM to investigate its interaction with . suspendability in TA solution increased with increasing diameter without the aid of sonication. Sorption affinity of for TA increased with decreasing diameter, positively related to their surface area. A two-stage sorption model was proposed to illustrate the interaction between and TA. TA molecules may be adsorbed first onto with aromatic rings to the surface carbon rings via -interactions, until forming a monolayer; the TA monolayer then further sorbed the dissolved TA by hydrogen bonds and other polar interactions. The sorbed TA increased the steric repulsion between individual , which might disperse the relatively loose aggregates and result in the stabilization of large-diameter in TA solution. The sorption and suspending processwere also examined bytransmission electron microscopy, providing further evidence for the above proposed -TA interactions. This study implies that widely distributed TA may promote the mobility and of in natural aqueous environments.
|
| [164] |
DOI:10.1021/es100598h
PMID:20687602
URL
[Cite within: 1]
Abstract The ever-increasing use of engineered nanomaterials will lead to heightened levels of these materials in the environment. The present review aims to provide a comprehensive overview of current knowledge regarding nanoparticle transport and aggregation in aquatic environments. Nanoparticle aggregation and deposition behavior will dictate particle transport potential and thus the environmental fate and potential ecotoxicological impacts of these materials. In this review, colloidal forces governing nanoparticle deposition and aggregation are outlined. Essential equations used to assess particle-particle and particle-surface interactions, along with Hamaker constants for specific nanoparticles and the attributes exclusive to nanoscale particle interactions, are described. Theoretical and experimental approaches for evaluating nanoparticle aggregation and deposition are presented, and the major findings of laboratory studies examining these processes are also summarized. Finally, we describe some of the challenges encountered when attempting to quantify the transport of nanoparticles in aquatic environments.
|
| [165] |
DOI:10.1021/es801641v
PMID:19068812
URL
[Cite within: 1]
Deposition of nanomaterials onto surfaces is a key process governing their transport, fate, and reactivity in aquatic systems. We evaluated the transport and deposition behavior of carboxyl functionalized single-walled carbon nanotubes (SWNTs) in a well-defined porous medium composed of clean quartz sand over a range of solution chemistries. Our results showthat increasing solution ionic strength or addition of calcium ions result in increased SWNT deposition (filtration). This observation is consistent with conventional colloid deposition theories, thereby suggesting that physicochemical filtration plays an important role in SWNT transport. However, the relatively insignificant change of SWNT filtration at low ionic strengths (< or = 3.0 mM KCl) and the incomplete breakthrough of SWNTs in deionized water (C/Co = 0.90) indicate that physical straining also plays a role in the capture of SWNTs within the packed sand column. It is proposed that SWNT shape and structure, particularly the very large aspect ratio and its highly bundled (aggregated) state in aqueous solutions, contribute considerably to straining in flow through porous media. We conclude that both physicochemical filtration and straining play a role at low (< 3.0 mM) ionic strength, while physicochemical filtration is the dominant mechanism of SWNT filtration at higher ionic strengths. Our results further show that deposited SWNTs are mobilized (released) from the quartz sand upon introduction of low ionic strength solution following deposition experiments with monovalent salt (KCl). In contrast, SWNTs deposited in the presence of calcium ions were not released upon introduction of low ionic strength solution to the packed column, even when humic acid was present in solution during SWNT deposition.
|
| [166] |
DOI:10.1021/es300839e
PMID:22582927
URL
[Cite within: 1]
Increasing use of engineered nanomaterials with novel properties relative to their bulk counterparts has generated a need to define their behaviors and impacts in the environment. The high surface area to volume ratio of nanoparticles results in highly reactive and physiochemically dynamic materials in environmental media. Many transformations, e.g. reactions with biomacromolecules, redox reactions, aggregation, and dissolution, may occur in both environmental and biological systems. These transformations and others will alter the fate, transport, and toxicity of nanomaterials. The nature and extent of these transformations must be understood before significant progress can be made toward understanding the environmental risks posed by these materials.
|
| [167] |
|
| [168] |
DOI:10.1016/j.chemosphere.2011.12.042
PMID:22245077
Magsci
URL
[Cite within: 1]
The increasing utilization of silver nanoparticles in industrial and consumer products has raised concern to wastewater treatment utilities due to its antimicrobial activity. In this work, the removal of citrate stabilized silver nanoparticles (Ag-NPs) during the wastewater treatment processes and its impact on treatment performance were examined. During simulated primary clarification, over 90% of the Ag-NPs remained in the wastewater, indicating that the majority of silver nanoparticles in sewage would enter the subsequent treatment units. During sequencing batch reactor processes, silver nanoparticles were effectively removed in each cycle throughout the 15-d experimental duration. Continuous input of silver nanoparticles into the wastewater did not significantly alter chemical oxygen demand (COD) removal. NH 4 removal was reduced at the beginning of the SBR experiment but quickly recovered at the later stage of the experiment. This study demonstrated that in the near future it is unlikely that citrate-stabilized Ag-NPs released into sewage will cause significant adversary effects on the COD and NH 4 removal of activated sludge processes in municipal wastewater treatment plants.
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
DOI:10.1089/ees.2012.0340
Magsci
URL
[Cite within: 3]
Engineered nanomaterials (ENMs) already occur in sewage and wastewater biosolids due to their release from commercial products (e. g., nanoscale titanium dioxide). Increasing levels and diversity of nanomaterials may enter sewage and wastewater treatment plants (WWTPs) in the future as they are released from products containing nanomaterials (e. g., coatings) embedded in products, or from industrial processes that use nanomaterials (e. g., polishing). Some metallic nanomaterials may dissolve (e. g., silver-, zinc-, or copper-based) or biodegrade (e. g., fullerenes) in wastewater, and subsequently sorb to settable biomass, precipitate as inorganic solids, or form stable aqueous complexes. Nanomaterials themselves sorb onto bacterial biomass in WWTPs, leading to their removal from water, but accumulation in biosolids that are disposed to land surface spreading fields, landfills, or incineration where their fate needs to be further considered. Because of the dense biological communities in WWTP unit processes, under typical conditions, >90% of the nanomaterials may attach to biomass, which is removed within the WWTP. Inclusion of membrane filtration to augment gravity settling has the potential to increase nanoparticle removals. At expected production/use levels, the presence of nanomaterials in biomass appears unlikely to influence current biosolids treatment processes (e. g., anaerobic digestion) or landfill biogas production. Additional research is needed to be able to monitor the transformation and removal of nanomaterials throughout WWTPs and biosolids treatment to assure they are not released into the environment where they may pose human or ecological risks.
|
| [173] |
|
| [174] |
DOI:10.1089/ees.2013.0472
Magsci
URL
[Cite within: 1]
In this study, we present a method for determining the relative affinity of nanoparticles (NPs) for an ensemble of other particles in a complex, heterogeneous suspension. We evaluated this method for NPs heteroaggregating with suspended solids present in activated sludge. A relationship was derived between the heterogeneous affinity coefficient, alpha, and measurements over time of the distribution coefficient, gamma, of NPs measured in supernatant versus those removed by heteroaggregation and subsequent settling. Application of this method, which uses a mathematical relationship to determine alpha from experimentally measured gamma values, to a series of metal and metal oxide NPs heteroaggregated with activated sludge indicated a relative affinity in the order of pristine CeO2, TiO2 NPs, and ZnO NPs > Ag(0) NPs surface-modified with polyvinylpyrrolidone (PVP) > citrate-functionalized CeO2 NP > Ag(0) NPs surface-modified with gum arabic. This trend in relative affinity followed the observed trend in removal such that higher affinity corresponded to higher removals of NPs. Values of alpha were calculated from measured relative affinities using average diameter and concentration of the activated sludge particles. The value calculated for PVP-stabilized Ag(0) NPs was comparable to a value previously reported for the attachment of these same NPs to a biofilm. Calculations also yielded size dependence of alpha in the case of the two Ag(0) evaluated that may be linked to NP dissolution.
|
| [175] |
URL
[Cite within: 1]
Advancement of the nanotechnology industry in Australia has seen numerous researchers beginning to handle nanomaterials, as well as the establishment of industrial facilities that are producing nanomaterials for incorporation into consumer products. Traditionally, the risk assessment of chemicals relies heavily on their composition, whereas the key determinants in the adverse effects caused by nanomaterials are their physical parameters (particle size, surface area and surface chemistry). At the present time, workplace exposure standards are not available, and appropriate methods that accurately characterise exposure to nanomaterials have not been established. Exposure of workers should therefore be "as low as reasonably practicable" through risk management programs that broadly encompass all of the hierarchy of controls used for ultrafine particulates, namely appropriate engineering controls, administrative controls and personal protective equipment.
|
| [176] |
DOI:10.1016/S0955-2219(02)00289-3
URL
[Cite within: 1]
Toxic lead-rich solid industrial wastes were stabilized by the vitrification method. Vitrification was attained by the addition of SiO 2 and Na 2O as vitrifying and melting agent, respectively. The non-toxic, homogeneous, vitreous products studied in the present work, contain 60 wt.% of solid waste. Products with such a high content of solid waste comprise an economically realistic suggestion, but are easily devitrified in conditions of large-scale production due to the difficulty to achieve rapid cooling conditions in the whole volume of a large piece of stabilized product. Thus, it must be ascertained that the loss of homogeneity is not accompanied with the loss of chemical stability. Differential Thermal Analysis (DTA) was applied in order to inspect the prospect to crystal phase separation. The separated crystal phases were characterized by X-ray diffraction and transmission electron microscopy. Possible devitrification processes are investigated in order to interconnect the microstructure with the chemical stability of the devitrified products.
|
| [177] |
URL
[Cite within: 1]
ABSTRACT Communities are reluctant to embrace new technologies where there is inadequate disclosure about the potential impacts on health or safety. Community outrage surrounding the recent widespread use of asbestos, despite the known health impacts, has led to distrust and scepticism towards new and emerging technologies which are heralded as revolutionary. Molecular genetics and nanotechnology are two examples of such technologies which have generated extensive debate regarding potential public and environmental health impacts. For nanotechnology, given the potential for toxicity and lack of characterisation data, the management of nanowaste will need to be addressed before community acceptance of the potential benefits of nanotechnology can occur. This paper evaluates current knowledge on methods of nanowaste containment and disposal; identifies the potential impact of uncontrolled release of nanoparticles from research and manufacturing facilities, as well as from consumer nanoproducts; and reviews current and potential methods of nanowaste containment and disposal among nanotechnology research and manufacturing facilities. Several types of controls are described in the literature for managing nanowaste, however these are not prescribed in any regulatory framework worldwide. Exposure from nanowaste streams would suggest that research urgently examine whether such controls are adequate, given the potential for occupational, public and environmental exposures.
|
| [178] |
DOI:10.1007/s11557-013-0933-3
URL
[Cite within: 1]
Nanoparticles (NPs) could reach the food chain from diverse wastes containing these potentially toxic substances. We studied the mycoextraction of alumina (Al 2 O 3 ) NPs by mycelia of edible fungi: Pleurotus eryngii and Trametes versicolor . Mycelia were cultivated in liquid medium supplemented with alumina nanoparticles (concentrations 0.001–0.102mol02L 611 ) to investigate accumulation of metal in the mycelium. The accumulation of Al in the mycelium depended on the duration of exposure, biomass of the mycelium and concentration of NPs. The efficiency of alumina-NP removal from the medium depended only on the duration of exposure and the fungal biomass, but not on NP concentration. Live hyphae of P. eryngii were more efficient in the removal of the NPs (658602% of total amount of NPs removed from medium) than T. versicolor (6102%). Dead mycelium of P. eryngii was less efficient (5102%), but also useful in the mycoextraction. These results were confirmed by scanning and transmission electron microscopy and laser ablation inductively coupled plasma mass spectrometry. Additionally, it was found that the mycoextraction efficiency by P. eryngii depended on NP type and was lower for NPs other than alumina: platinum – 5802% and cobalt – 1302%.
|
| [179] |
|
| [180] |
|
| [181] |
|
| [182] |
|
| [183] |
|
| [184] |
DOI:10.1016/S0043-1354(02)00616-4
PMID:12691889
URL
[Cite within: 1]
In this study we present a new approach to determine volumes, heterogeneity factors, and compositions of the bacterial population of activated sludge flocs by 3D confocal imaging. After staining the fresh flocs with fluorescein-isothiocyanate, 75 stacks of images (containing approx. 3000 flocs) were acquired with a confocal laser scanning microscope. The self-developed macro 3D volume and surface determination for the KS 400 software package combined the images of one stack to a 3D image and calculated the real floc volume and surface. We determined heterogeneity factors like the ratio of real floc surface to the surface of a sphere with the respective volume and the fractal dimension (D(f)). According to their significant influence on floc integrity and quality, we also investigated the chemical composition of flocs and quantified their bacterial population structure by using group-specific rRNA-targeted probes for fluorescence in situ hybridization. By a settling experiment we enriched flocs with poor settling properties and determined the above-mentioned parameters. This approach revealed shifts in floc volume, heterogeneity, and bacterial and chemical composition according to the settling quality of the flocs.
|
| [185] |
DOI:10.1016/S0273-1223(97)00447-2
URL
[Cite within: 1]
ABSTRACT Activated sludge is the most widely used process for wastewater treatment. However basic properties such as floc structure or settling properties, still remain unknown. In order to describe activated sludge floc structure, four investigation methods were used: (i) size distribution analysis associated with sonication, (ii) transmission electron microscopy (iii) laser scanning confocal microscopy and fractal dimension analysis, and (iv) three dimensional modelling.The evolution of floc size distribution in dispersed samples was used to build a model of floc showing that the predominating microflocs (125 08m) are formed from 13 08m aggregates, which are made up of smaller particles (2.5 08m). By transmission electron microscopy it was assumed that the 2.5 08m sub-units could correspond to microorganisms and that exopolymers form a gel-like matrix which holds the structure of the aggregates together.The microfloc (13 08m) structure was investigated by confocal scanning light microscopy and image processing. The analysis of the mass distribution pointed out that the microfloc is a fractal object (fractal dimension around 3). The total number of cells in these aggregates was found to be 2n number. These two results suggest that division of microorganisms explained the formation of 13 08m units which are microcolonies.A mass fractal dimension 2.5 ± 0.15 was measured for the microfloc. Its external surface was reconstructed by using interpolating software (GOCAD). It appeared that the surface is also a fractal object with a dimension 2.3 ± 0.1. According to the fractal theory, it seems that diffusion limited aggregation via monomer-cluster collision is the mechanism which describes the formation of macroflocs.
|
| [186] |
DOI:10.1016/j.scitotenv.2007.10.010
PMID:18031795
URL
[Cite within: 2]
Products with antimicrobial effect based on silver nanoparticles are increasingly used in Asia, North America and Europe. This study presents an analysis of risk to freshwater ecosystems from silver released from these nanoparticles incorporated into textiles and plastics. The analysis is presented in four stages; (i) silver mass flow analysis and estimation of emissions, (ii) assessment of the fate of silver in a river system and estimation of predicted environmental concentrations (PECs), (iii) critical evaluation of available toxicity data for environmentally relevant forms of silver and estimation of predicted no-effect concentrations (PNECs), and (iv) risk characterization. Our assessment is based on estimated silver use in the year 2010, focusing on the Rhine river as a case study. In 2010, biocidal plastics and textiles are predicted to account for up to 15% of the total silver released into water in the European Union. The majority of silver released into wastewater is incorporated into sewage sludge and may be spread on agricultural fields. The amount of silver reaching natural waters depends on the fraction of wastewater that is effectively treated. Modeled PECs in the Rhine river are in satisfactory agreement with monitoring data from other river systems. Because a complete characterization of the toxicity of environmentally relevant silver species is lacking, only a limited risk assessment is possible at this time. However, our study indicates that PEC/PNEC ratios greater than 1 cannot be ruled out for freshwater ecosystems, in particular sediments. No risk is predicted for microbial communities in sewage treatment plants.
|
| [187] |
|
| [188] |
DOI:10.1021/es101565j
PMID:20839838
URL
[Cite within: 1]
Nanosized silver sulfide (伪-Ag(2)S) particles were identified in the final stage sewage sludge materials of a full-scale municipal wastewater treatment plant using analytical high-resolution transmission electron microscopy. The Ag(2)S nanocrystals are in the size range of 5-20 nm with ellipsoidal shape, and they form very small, loosely packed aggregates. Some of the Ag(2)S nanoparticles (NPs) have excess S on the surface of the sulfide minerals under S-rich environments, resulting in a ratio of Ag to S close to 1. Considering the current extensive production of Ag NPs and their widespread use in consumer products, it is likely that they are entering wastewater streams and the treatment facilities that process this water. This study suggests that in a reduced, S-rich environment, such as the sedimentation processes during wastewater treatment, nanosized silver sulfides are being formed. This field-scale study provides for the first time nanoparticle-level information of the Ag(2)S present in sewage sludge products, and further suggests the role of wastewater treatment processes on transformation of Ag nanoparticles and ionic Ag potentially released from them.
|
| [189] |
|
| [190] |
DOI:10.1021/es901102n
PMID:19764246
URL
[Cite within: 3]
Abstract Titanium (Ti) occurs naturally in soils and as highly purified titanium dioxide (Ti5O2) in many commercial products that have been used for decades. We report for the first time the occurrence, characterization, and removal of nano- and larger-sized Ti at wastewater treatment plants (WWTPs). At one WWTP studied in detail, raw sewage contained 100 to nearly 3000 microg TVL Ti larger than 0.7 microm accounted for the majority of the Ti in raw sewage, and this fraction was well removed by WWTP processes. Ti concentrations in effluents from this and several other WWTPs ranged from <5 to 15 microg/L and were nearly all present in the < 0.7 microm size fraction. As Ti was removed, it accumulated in settled solids at concentrations ranging from 1 to 6 microg Ti/mg. Ti-containing solids were imaged in sewage, biosolids, and liquid effluent as well as in commercial products containing engineered TiO2. Single nanoparticles plus spherical aggregates (50 nm to a few hundred nanometer in size) composed of sub-50 nm spheres of Ti and oxygen only (presumably TiO2) were observed in all samples. Significantly larger silicate particles containing a mixture of Ti and other metal atoms were also observed in the samples. To support the field work, laboratory adsorption batch and sequencing batch reactor experiments using TiO2 and activated sludge bacteria verified that adsorption of TiO2 onto activated sludge biomass occurs. Monitoring for TiO2 in the environment where WWTP liquid effluent is discharged (rivers, lakes, oceans) or biomass disposed (landfills, agriculture and soil amendments, incinerator off-gas or residuals) will increase our knowledge on the fate and transport of other nanomaterials in the environment
|
| [191] |
DOI:10.2217/nnm.10.61
PMID:20735233
URL
Among all environmental contaminants, those emerging from nanotechnologies constitute one of the most critical challenges for the coming years. The new properties of nanoparticles are at the heart of current scientific advances and the growing interest in harnessing them brings awareness of potential impacts that we cannot ignore. To date, scientists and industrialists have focused on the manufacture of nanomaterials more than on the assessment of the risks for humans and ecosystems. Few databases exist regarding the amounts released within ecosystems and no specific procedure of recycling has yet been established. However, nanoparticles cannot be considered as molecular pollutants or larger particles, and careful consideration is needed to establish a legal system that is specific. Their novel properties, surface energy and reactivity make it impossible to simply transfer our physicochemical, thermodynamic and toxicological knowledge from the micronscale to the nanoscale. This article highlights, nonexhaustively, the strong relationship existing between the unique properties of metallic and metal oxide nanoparticles and their biological effects on aquatic organisms.
|
| [192] |
DOI:10.1021/es903757q
PMID:20222656
URL
[Cite within: 1]
A number of commercialized nanomaterials incorporate TiO(2) nanoparticles. Studying their structural stability in media mimicking the environment or the conditions of use is crucial in understanding their potential eco-toxicological effects. We focused here on a hydrophobic TiO(2) nanoparticle-based formulation used in cosmetics: T-Lite SF. It is composed of a TiO(2) core, coated with two successive protective layers of Al(OH)(3), and polydimethylsiloxane. Soon after contact with water (pH = 5, low ionic strength), the T-Lite SF becomes hydrophilic and form aggregates. During this aging, 90%wt of the total Si of the organic layer is desorbed, and the PDMS remaining at the surface is oxidized. The Al(OH)(3) layer is also affected but remains sorbed at the surface. This remaining Al-based layer still protects from the production of superoxide ions from the photoactive/phototoxic TiO(2) core in our experimental conditions.
|
| [193] |
DOI:10.1089/ees.2012.0340
Magsci
URL
[Cite within: 1]
Engineered nanomaterials (ENMs) already occur in sewage and wastewater biosolids due to their release from commercial products (e. g., nanoscale titanium dioxide). Increasing levels and diversity of nanomaterials may enter sewage and wastewater treatment plants (WWTPs) in the future as they are released from products containing nanomaterials (e. g., coatings) embedded in products, or from industrial processes that use nanomaterials (e. g., polishing). Some metallic nanomaterials may dissolve (e. g., silver-, zinc-, or copper-based) or biodegrade (e. g., fullerenes) in wastewater, and subsequently sorb to settable biomass, precipitate as inorganic solids, or form stable aqueous complexes. Nanomaterials themselves sorb onto bacterial biomass in WWTPs, leading to their removal from water, but accumulation in biosolids that are disposed to land surface spreading fields, landfills, or incineration where their fate needs to be further considered. Because of the dense biological communities in WWTP unit processes, under typical conditions, >90% of the nanomaterials may attach to biomass, which is removed within the WWTP. Inclusion of membrane filtration to augment gravity settling has the potential to increase nanoparticle removals. At expected production/use levels, the presence of nanomaterials in biomass appears unlikely to influence current biosolids treatment processes (e. g., anaerobic digestion) or landfill biogas production. Additional research is needed to be able to monitor the transformation and removal of nanomaterials throughout WWTPs and biosolids treatment to assure they are not released into the environment where they may pose human or ecological risks.
|
| [194] |
DOI:10.1016/j.watres.2010.05.036
PMID:20547403
Magsci
URL
[Cite within: 4]
Sorption to activated sludge is a major removal mechanism for pollutants, including manufactured nanoparticles (NPs), in conventional activated sludge wastewater treatment plants. The objectives of this work were to (1) image sorption of fluorescent NPs to wastewater biomass; (2) quantify and compare biosorption of different types of NPs exposed to wastewater biomass; (3) quantify the effects of natural organic matter (NOM), extracellular polymeric substances (EPS), surfactants, and salt on NP biosorption; and (4) explore how different surface functionalities for fullerenes affect biosorption. Batch sorption isotherm experiments were conducted with activated sludge as sorbent and a total of eight types of NPs as sorbates. Epifluorescence images clearly show the biosorption of fluorescent silica NPs; the greater the concentration of NPs exposed to biomass, the greater the quantity of NPs that biosorb. Furthermore, biosorption removes different types of NPs from water to different extents. Upon exposure to 400 mg/L total suspended solids (TSS) of wastewater biomass, 97% of silver nanoparticles were removed, probably in part by aggregation and sedimentation, whereas biosorption was predominantly responsible for the removal of 88% of aqueous fullerenes, 39% of functionalized silver NPs, 23% of nanoscale titanium dioxide, and 13% of fullerol NPs. Of the NP types investigated, only aq-Cshowed a change in the degree of removal when the NP suspension was equilibrated with NOM or when EPS was extracted from the biomass. Further study of carbonaceous NPs showed that different surface functionalities affect biosorption. Thus, the production and transformations in NP surface properties will be key factors in determining their fate in the environment.
|
| [195] |
DOI:10.1021/es1041892
PMID:21466186
URL
[Cite within: 1]
We investigated the behavior of metallic silver nanoparticles (Ag-NP) in a pilot wastewater treatment plant (WWTP) fed with municipal wastewater. The treatment plant consisted of a nonaerated and an aerated tank and a secondary clarifier. The average hydraulic retention time including the secondary clarifier was 1 day and the sludge age was 14 days. Ag-NP were spiked into the nonaerated tank and samples were collected from the aerated tank and from the effluent. Ag concentrations determined by inductively coupled plasma-mass spectrometry (ICP-MS) were in good agreement with predictions based on mass balance considerations. Transmission electron microscopy (TEM) analyses confirmed that nanoscale Ag particles were sorbed to wastewater biosolids, both in the sludge and in the effluent. Freely dispersed nanoscale Ag particles were only observed in the effluent during the initial pulse spike. X-ray absorption spectroscopy (XAS) measurements indicated that most Ag in the sludge and in the effluent was present as Ag(2)S. Results from batch experiments suggested that Ag-NP transformation to Ag(2)S occured in the nonaerated tank within less than 2 h. Physical and chemical transformations of Ag-NP in WWTPs control the fate, the transport and also the toxicity and the bioavailability of Ag-NP and therefore must be considered in future risk assessments.
|
| [196] |
|
| [198] |
DOI:10.1016/j.biortech.2011.12.113
PMID:22265985
URL
[Cite within: 1]
This study investigated the fate of nano-CeO(2) during municipal wastewater treatment using a laboratory-scale activated sludge (A/S) system fed with primarily-treated municipal wastewater and nano-CeO(2) (55.0 mg Ce/L). Nano-CeO(2) was highly removed during A/S treatment (96.6% total Ce). Extensive removal of CeO(2) <200 nm was also attained and the concentration escaping treatment was only 0.11 mg Ce/L. Elimination occurred mainly by aggregation and settling of CeO(2) particles, promoted by circumneutral pH values and by nanoparticle interactions with organic and/or inorganic wastewater constituents. Biosorption also contributed to CeO(2) removal as shown by sludge analysis and batch adsorption studies. Batch bioassays demonstrated that nano-CeO(2) only exerted inhibition of by A/S at concentrations exceeding those in the bioreactor feed (50% inhibition at 950 mg CeO(2)/L). These findings indicate that A/S treatment is expected to provide extensive removal of nano-CeO(2) in municipal wastewaters.
|
| [197] |
DOI:10.1016/j.jhazmat.2011.10.086
PMID:22154869
URL
[Cite within: 1]
As engineered nanomaterials (NMs) become used in industry and commerce their loading to sewage will increase. In this research, sequencing batch reactors (SBRs) were operated with hydraulic (HRT) and sludge (SRT) retention times representative of full-scale biological WWTPs for several weeks. Under environmentally relevant NM loadings and biomass concentrations, NMs had negligible effects on ability of the wastewater bacteria to biodegrade organic material, as measured by chemical oxygen demand (COD). Carboxy-terminated polymer coated silver nanoparticles ( fn-Ag) were removed less effectively (88% removal) than hydroxylated fullerenes (fullerols; >90% removal), nano TiO 2 (>95% removal) or aqueous fullerenes ( nC 60; >95% removal). Experiments conducted over 4 months with daily loadings of nC 60 showed that nC 60 removal from solution depends on the biomass concentration. Under conditions representative of most suspended growth biological WWTPs (e.g., activated sludge), most of the NMs will accumulate in biosolids rather than in liquid effluent discharged to surface waters. Significant fractions of fn-Ag were associated with colloidal material which suggests that efficient particle separation processes (sedimentation or filtration) could further improve removal of NM from effluent.
|
| [199] |
DOI:10.1039/b926029c
URL
[Cite within: 2]
Detection and characterisation are two of the major challenges in understanding the fate, behaviour and occurrence of engineered nanoparticles (ENPs) in the natural environment. In a previous paper we described the development of hydrodynamic chromatography coupled to plasma mass spectrometry (HDC-ICP-MS) for detecting and characterising ENPs in aqueous matrices. This paper describes the applicability of the approach, to study the behaviour of silver nanoparticles in a much more complex and relevant environmental systemi.e.sewage sludge supernatant. Batch sorption studies were performed at a range of nanosilver concentrations. Following completion, the sludge supernatant was characterised by ICP-MS, HDC-ICP-MS and transmission electron microscopy (TEM). It was found that, after a contact time of 6 h, most of the silver had partitioned to the sewage sludge (>90%). However, of the silver remaining in the supernatant, some of this was in the nanoparticle form, implying that closer consideration should be given to the longer-term impact of the release of silver ENPs into aquatic ecosystems. These preliminary data clearly show the utility of HDC-ICP-MS for studying the occurrence and behaviour of ENPs in complex natural environments.
|
| [200] |
DOI:10.1021/es901399q
PMID:20028062
URL
[Cite within: 1]
Through novel application of small-angle neutron scattering, we examined the fate of silica nanoparticles (SiO(2)NPs) during simulated primary wastewater treatment, by measuring, in real time, the colloidal behavior of SiO(2)NPs in wastewater (sewage). We examined the effects of surface functionality on SiO(2)NP fate in wastewater, by comparing both unfunctionalized (uncoated or "bare") SiO(2)NPs and SiO(2)NPs functionalized with a thin coating of a nonionic surfactant (Tween 20), which is widely used in personal care and household product formulations containing engineered oxide nanoparticles. Our results show new evidence that the surface functionality of SiO(2)NPs plays a crucial role in their flocculation and sedimentation behavior in wastewater, and thus the likely efficacy of their removal from the effluent stream during primary wastewater treatment. Uncoated SiO(2)NPs did not flocculate in wastewater over typical residence times for primary treatment. Conversely, surface-functionalized (Tween-coated) SiO(2)NPs underwent rapid flocculation in wastewater. Our results show that the surface-functionalized SiO(2)NPs are likely to be removed by sedimentation to sewage sludge (typically recycled to land), whereas uncoated SiO(2)NPs will continue through the effluent stream. While nanoparticle design is driven by use purpose, this study shows new potential for exploiting surface functionalization of nanoparticles to modify their environmental pathways.
|
| [201] |
DOI:10.1021/es404946y
PMID:24870403
URL
[Cite within: 2]
Engineered nanomaterials (ENMs) are used to enhance the properties of many manufactured products and technologies. Increased use of ENMs will inevitably lead to their release into the environment. An important route of exposure is through the waste stream, where ENMs will enter wastewater treatment plants (WWTPs), undergo transformations, and be discharged with treated effluent or biosolids. To better understand the fate of a common ENM in WWTPs, experiments with laboratory-scale activated sludge reactors and pristine and citrate-functionalized CeO2 nanoparticles (NPs) were conducted. Greater than 90% of the CeO2 introduced was observed to associate with biosolids. This association was accompanied by reduction of the Ce(IV) NPs to Ce(III). After 5 weeks in the reactor, 44 卤 4% reduction was observed for the pristine NPs and 31 卤 3% for the citrate-functionalized NPs, illustrating surface functionality dependence. Thermodynamic arguments suggest that the likely Ce(III) phase generated would be Ce2S3. This study indicates that the majority of CeO2 NPs (>90% by mass) entering WWTPs will be associated with the solid phase, and a significant portion will be present as Ce(III). At maximum, 10% of the CeO2 will remain in the effluent and be discharged as a Ce(IV) phase, governed by cerianite (CeO2).
|
| [202] |
DOI:10.1016/j.scitotenv.2014.12.056
PMID:255851562
URL
[Cite within: 2]
A model for nanoparticle (NP) separation and transformation in water treatment was parameterized for three metal and metal-oxide NPs. Functional assays to determine NP specific distribution and transformation were used to parameterize the model and obtain environmentally relevant concentrations of NPs and transformation byproducts leaving WWTPs in effluent and biosolids. All three NPs were predicted to associate >90% with the solid phase indicating significant accumulation in the biosolids. High rates of transformation for ZnO and Ag NPs resulted in ~97% transformation of the NPs that enter the plant despite differences in transformation rate in aerobic versus anaerobic environments. Due to high insolubility and negligible redox transformation, the only process predicted to impact TiO 2 NP fate and transport in WWTPs was distribution between the solid and liquid phases. Subsequent investigation of ZnO NP species fate and transport when land applied in biosolids indicated that steady state concentrations of ZnO phases would likely be achieved after approximately 150days under loading conditions of biosolids typical in current practice.
|
| [203] |
DOI:10.1089/ees.2013.0472
Magsci
URL
[Cite within: 2]
In this study, we present a method for determining the relative affinity of nanoparticles (NPs) for an ensemble of other particles in a complex, heterogeneous suspension. We evaluated this method for NPs heteroaggregating with suspended solids present in activated sludge. A relationship was derived between the heterogeneous affinity coefficient, alpha, and measurements over time of the distribution coefficient, gamma, of NPs measured in supernatant versus those removed by heteroaggregation and subsequent settling. Application of this method, which uses a mathematical relationship to determine alpha from experimentally measured gamma values, to a series of metal and metal oxide NPs heteroaggregated with activated sludge indicated a relative affinity in the order of pristine CeO2, TiO2 NPs, and ZnO NPs > Ag(0) NPs surface-modified with polyvinylpyrrolidone (PVP) > citrate-functionalized CeO2 NP > Ag(0) NPs surface-modified with gum arabic. This trend in relative affinity followed the observed trend in removal such that higher affinity corresponded to higher removals of NPs. Values of alpha were calculated from measured relative affinities using average diameter and concentration of the activated sludge particles. The value calculated for PVP-stabilized Ag(0) NPs was comparable to a value previously reported for the attachment of these same NPs to a biofilm. Calculations also yielded size dependence of alpha in the case of the two Ag(0) evaluated that may be linked to NP dissolution.
|
| [204] |
DOI:10.1021/es800091f
PMID:18754516
URL
[Cite within: 1]
The rapidly increasing production of engineered nanoparticles has created a demand for particle removal from industrial and communal wastewater streams. Efficient removal is particularly important in view of increasing long-term persistence and evidence for considerable ecotoxicity of specific nanoparticles. The present work investigates the use of a model wastewater treatment plant for removal of oxide nanoparticles. While a majority of the nanoparticles could be captured through adhesion to clearing sludge, a significant fraction of the engineered nanoparticles escaped the wastewater plant's clearing system, and up to 6 wt % of the model compound cerium oxide was found in the exit stream of the model plant. Our study demonstrates a significant influence of surface charge and the addition of dispersion stabilizing surfactants as routinely used in the preparation of nanoparticle derived products. A detailed investigation on the agglomeration of oxide nanoparticles in wastewater streams revealed a high stabilization of the particles against clearance (adsorption on the bacteria from the sludge). This unexpected finding suggests a need to investigate nanoparticle clearance in more detail and demonstrates the complex interactions between dissolved species and the nanoparticles within the continuously changing environment of the clearing sludge.
|
| [205] |
DOI:10.1016/j.scitotenv.2011.03.022
PMID:21529894
Magsci
URL
[Cite within: 1]
The release of nanoparticles (NPs) into the environment, including wastewater treatment plants, is expected to increase in the future. Therefore, it is important to understand the potential effects of these NPs on activated sludge treatment processes. A pulse-flow respirometer was used to study the toxicity of nano-ZnO on activated sludge endogenous respiration, BOD biodegradation, and nitrification. In addition, toxicities of bulk ZnO particles and Zn ion (e.g. soluble Zn) were also studied. All three Zn forms were found to adversely impact the activity of activated sludge, with soluble Zn exhibited the greatest toxicity. The effects of nano-ZnO and bulk ZnO on activated sludge were caused by soluble Zn resulting from ZnO particle dissolution. The IC(50) values of soluble Zn on activated sludge endogenous respiration, BOD biodegradation, ammonia oxidation, and nitrite oxidation were 2.2, 1.3, 0.8, and 7.3 mg-Zn/L, respectively. Therefore, the first step of nitrification was most sensitive to Zn.
|
| [206] |
DOI:10.1186/1752-153X-7-46
PMID:23497481
URL
Manufactured silver nanoparticles (AgNPs) are one of the most commonly used nanomaterials in consumer goods and consequently their concentrations in wastewater and hence wastewater treatment plants ar
|
| [207] |
|
| [208] |
DOI:10.1021/es2007758
PMID:21598969
URL
[Cite within: 1]
Despite the increasing use of silver nanoparticles (Ag-NPs) in nanotechnology and their toxicity to invertebrates, the transformations and fate of Ag-NPs in the environment are poorly understood. This work focuses on the sulfidation processes of PVP-coated Ag-NPs, one of the most likely corrosion phenomena that may happen in the environment. The sulfur to Ag-NPs ratio was varied in order to control the extent of Ag-NPs transformation to silver sulfide (Ag60S). A combination of synchrotron-based X-ray Diffraction (XRD) and Extended X-ray Absorption Fine Structure spectroscopy shows the increasing formation of Ag60S with an increasing sulfur to Ag-NPs ratio. TEM observations show that Ag60S forms nanobridges between the Ag-NPs leading to chain-like structures. In addition, sulfidation strongly affects surface properties of the Ag-NPs in terms of surface charge and dissolution rate. Both may affect the reactivity, transport, and toxicity of Ag-NPs in soils. In particular, the decrease of dissolution rate as a function of sulfide exposure may strongly limit Ag-NPs toxicity since released Ag62 ions are known to be a major factor in the toxicity of Ag-NPs.
|
| [209] |
|
| [210] |
DOI:10.1021/es301487s
PMID:22816872
URL
[Cite within: 2]
The rapid development and commercialization of nanomaterials will inevitably result in the release of nanoparticles (NPs) to the environment. As NPs often exhibit physical and chemical properties significantly different from those of their molecular or macrosize analogs, concern has been growing regarding their fate and toxicity in environmental compartments. The wastewater-sewage sludge pathway has been identified as a key release pathway leading to environmental exposure to NPs. In this study, we investigated the chemical transformation of two ZnO-NPs and one hydrophobic ZnO-NP commercial formulation (used in personal care products), during anaerobic digestion of wastewater. Changes in Zn speciation as a result of postprocessing of the sewage sludge, mimicking composting/stockpiling, were also assessed. The results indicated that "native" Zn and Zn added either as a soluble salt or as NPs was rapidly converted to sulfides in all treatments. The hydrophobicity of the commercial formulation retarded the conversion of ZnO-NP. However, at the end of the anaerobic digestion process and after postprocessing of the sewage sludge (which caused a significant change in Zn speciation), the speciation of Zn was similar across all treatments. This indicates that, at least for the material tested, the risk assessment of ZnO-NP through this exposure pathway can rely on the significant knowledge already available in regard to other "conventional" forms of Zn present in sewage sludge.
|
| [211] |
DOI:10.1021/es060999b
PMID:17051814
URL
[Cite within: 1]
The production of nanoparticles (NPs) is increasing rapidly for applications in electronics, chemistry, and biology. This interest is due to the very small size of NPs which provides them with many interesting properties such as rapid diffusion, high specific surface areas, reactivity in liquid or gas phase, and a size close to biomacromolecules. In turn, these extreme abilities might be a problem when considering a potentially uncontrolled exposure to the environment. For instance, nanoparticles might be highly mobile and rapidly transported in the environment or inside the body through a water or air pathway. Accordingly, the very fast development of these new synthetic nanomaterials raises questions about their impact on the environment and human health. We have studied the impact of a model water dispersion of nanoparticles (7 nm CeO2 oxide) on a Gram-negative bacteria (Escherichia coli). The nanoparticles are positively charged at neutral pH and thus display a strong electrostatic attraction toward bacterial outer membranes. The counting of colony forming units (CFU) after direct contact with CeO2 NPs allows for the defining of the conditions for which the contact is lethal to Escherichia coli. Furthermore, a set of experiments including sorption isotherms, TEM microscopy, and X-ray absorption spectroscopy (XAS) at cerium L3 edge is linked to propose a scenario for the observed toxic contact.
|
| [212] |
DOI:10.3109/17435390903305260
URL
[Cite within: 1]
Physico-chemical interactions between nanoparticles and cell membranes play a crucial role in determining the cytotoxicity of nanoparticles, which may thereby vary depending on the nature of the target microorganisms. We investigated the responses of two different models of unicellular bacteria to cerium oxide (CeO2) nanoparticles. These organisms are: Synechocystis PCC6803 a representative of environmentally important cyanobacterial organisms (producer of biomass for aquatic food chains), and Escherichia coli a representative of intestine-colonizing bacteria. Coupling physico-chemical (adsorption isotherms and electrophoretic mobility), biological (survival tests), microscopical (SEM, TEM and EDS) and spectroscopic (XANES) methods, we enlightened two distinct mechanisms for the CeO2 nanoparticles toxicological impact: A 'direct' mechanism that requires a close contact between nanoparticles and cell membranes, and an 'indirect' influence elicited by the acidity of nanoparticles stabilizing agents. We showed that E. coli is sensitive to the 'direct' effects of nanoparticles, whereas Synechocystis being protected by extracellular polymeric substances preventing direct cellular contacts is sensitive only to the 'indirect' mechanism. Consequently, our findings demonstrate the importance of the 'direct/indirect' effects of nanoparticles on cell fitness, a phenomenon that should be systematically investigated with appropriate techniques and dose metrics to make meaningful environmental and/or health recommendations.
|
| [213] |
DOI:10.1021/es204540z
PMID:22533675
URL
[Cite within: 1]
Abstract Silver nanoparticles (AgNPs) are increasingly used as bacteriostatic agents to prevent microbial growth. AgNPs are manufactured with a variety of coatings, and their potential impacts on wastewater treatment in general are poorly understood. In the present study, Nitrosomonas europaea, a model ammonia oxidizing bacterium, was exposed to AgNPs with citrate, gum arabic (GA), and polyvinylpyrrolidone (PVP). GA and citrate AgNPs inhibited nitrification most strongly (67.9 3.6% and 91.4 0.2%, respectively at 2 ppm). Our data indicate that Ag(+) dissolution and colloid stability of AgNPs were the main factors in AgNP toxicity. In general, low amounts of dissolved Ag initially caused a post-transcriptional interruption of membrane-bound nitrifying enzyme function, reducing nitrification by 10% or more. A further increase in dissolved Ag resulted in heavy metal stress response (e.g., merA up-regulation) and ultimately led to membrane disruption. The highest effect on membrane disruption was observed for citrate AgNPs (64 11% membranes compromised at 2 ppm), which had high colloidal stability. This study demonstrates that coating plays a very important role in determining Ag dissolution and ultimately toxicity to nitrifiers. More research is needed to characterize these parameters in complex growth media such as wastewater.
|
| [214] |
DOI:10.1039/c2em30655g
PMID:24592426
URL
[Cite within: 2]
Abstract Metallic and metal oxide nanomaterials have been increasingly used in consumer products (e.g. sunscreen, socks), the medical and electronic industries, and environmental remediation. Many of them ultimately enter wastewater treatment plants (WWTPs) or landfills. This review paper discusses the fate and potential effects of four types of nanoparticles, namely, silver nanoparticles (AgNPs), nano ZnO, nano TiO2, and nano zero valent iron (NZVI), on waste/wastewater treatment and anaerobic digestion. The stabilities and chemical properties of these nanoparticles (NPs) result in significant differences in antimicrobial activities. Analysis of published data of metallic and metal oxide NPs suggests that oxygen is often a prerequisite for the generation of reactive oxygen species (ROS) for AgNPs and NZVI, while illumination is necessary for ROS generation for nano TiO2 and nano ZnO. Furthermore, such nanoparticles are capable of being oxidized or dissolved in water and can release metal ions, leading to metal toxicity. Therefore, AgNPs and nano TiO2 are chemically stable NPs that have no adverse effects on microbes under anaerobic conditions. Although the toxicity of nanomaterials has been studied intensively under aerobic conditions, more research is needed to address their fate in anaerobic waste/wastewater treatment systems and their long-term effects on the environment.
|
| [215] |
DOI:10.1016/j.jhazmat.2010.01.087
PMID:20149532
URL
[Cite within: 1]
The discharge of carbon nanotubes (CNTs) from industrial waste or disposal of such materials from commercial and/or domestic use will inevitably occur with increasing production and enter into wastewater treatment facilities with unknown consequences. Therefore, a better knowledge of the toxicity of CNTs to biological processes in wastewater treatment will be critical. This study examined the toxicity of multi-walled carbon nanotubes (MWCNTs) on the microbial communities in activated sludge. A comparative study using the activated sludge respiration inhibition test was performed on both unsheared mixed liquor and sheared mixed liquor to demonstrate the potential toxicity posed by MWCNTs and to illustrate the extent of extracellular polymeric substances (EPS) in protecting the microorganisms from the toxicity of CNTs. Respiration inhibition was observed for both unsheared and sheared mixed liquor when MWCNTs were present, however, greater respiration inhibition was observed for the sheared mixed liquor. The toxicity observed by the respiration inhibition test was determined to be dose-dependent; the highest concentration of MWCNTs exhibited the highest respiration inhibition. Scanning Electron Microscopy (SEM) images demonstrated direct physical contact between MWCNTs and activated sludge flocs.
|
| [216] |
DOI:10.1016/j.watres.2010.06.060
PMID:20638703
URL
[Cite within: 1]
The growing release of nanosilver into sewage systems has increased the concerns on the potential adverse impacts of silver nanoparticles (AgNPs) in wastewater treatment plants. The inhibitory effects of nanosilver on wastewater treatment and the response of activated sludge bacteria to the shock loading of AgNPs were evaluated in a Modified Ludzack-Ettinger (MLE) activated sludge treatment system. Before shock-loading experiments, batch extant respirometric assays determined that at 1mg/L of total Ag, nitrification inhibitions by AgNPs (average size=1-29 nm) and Ag(+) ions were 41.4% and 13.5%, respectively, indicating that nanosilver was more toxic to nitrifying bacteria in activated sludge than silver ions. After a 12-h period of nanosilver shock loading to reach a final peak silver concentration of 0.75 mg/L in the MLE system, the total silver concentration in the mixed liquor decreased exponentially. A continuous flow-through model predicted that the silver in the activated sludge system would be washed out 25 days after the shock loading. Meanwhile, a prolonged period of nitrification inhibition (>1 month, the highest degree of inhibition=46.5%) and increase of ammonia/nitrite concentration in wastewater effluent were observed. However, nanosilver exposure did not affect the growth of heterotrophs responsible for organic matter removal. Microbial community structure analysis indicated that the ammonium-oxidizing bacteria and nitrite-oxidizing bacteria, Nitrospira, had experienced population decrease while Nitrobacter was washed out after the shock loading.
|
| [217] |
DOI:10.1016/j.envpol.2008.10.002
PMID:19013699
URL
[Cite within: 2]
The level of production of nanoparticles will inevitably lead to their appearance in air, water, soils, and organisms. A theoretical framework that relates properties of nanoparticles to their biological effects is needed to identify possible risks to human health and the environment. This paper considers the properties of dispersed metallic nanoparticles and highlights the relationship between the chemical stability of these nanoparticles and their in vitro toxicity. Analysis of published data suggests that chemically stable metallic nanoparticles have no significant cellular toxicity, whereas nanoparticles able to be oxidized, reduced or dissolved are cytotoxic and even genotoxic for cellular organisms.
|
| [218] |
DOI:10.1038/nnano.2009.242
PMID:19809453
URL
[Cite within: 2]
The regulation of engineered nanoparticles requires a widely agreed definition of such particles. Nanoparticles are routinely defined as particles with sizes between about 1 and 100 nm that show properties that are not found in bulk samples of the same material. Here we argue that evidence for novel size-dependent properties alone, rather than particle size, should be the primary criterion in any definition of nanoparticles when making decisions about their regulation for environmental, health and safety reasons. We review the size-dependent properties of a variety of inorganic nanoparticles and find that particles larger than about 30 nm do not in general show properties that would require regulatory scrutiny beyond that required for their bulk counterparts.
|
| [219] |
DOI:10.1006/jcis.1998.5570
PMID:9698415
URL
[Cite within: 1]
The fractal dimension of a particle aggregate can provide fundamental information on the structure and origin of the aggregate. The analysis of large chemically homogeneous fractal objects has been achieved, but reliable methods of estimating the fractal dimensions of large and chemically heterogeneous aggregates are needed. To this end, we used confocal scanning laser microscopy in which thin optical sections of aggregates were obtained in order to calculate their 2D and ultimately 3D fractal dimensions according to the Mandelbrot theory. Fractal dimensions of 2.08 0.11 for a Brownian aggregation of latex particles and 2.25 0.12 for shear aggregation were determined using the confocal technique. These values are within the ranges for universality classes predicted for such aggregates and observed by previous investigators. Thus, this method appears to provide reliable estimates of the fractal dimension with particular utility in the characterization of aggregates composed of larger particles or complex materials where the fractal dimension may not be accessible by light-scattering measurements. The confocal method is used to analyze flocs of activated sludge material as one example of the application of this method to more complex, large (up to 500 m), and chemically heterogeneous flocs.
|
| [220] |
DOI:10.1016/j.envpol.2011.03.003
PMID:21481996
Magsci
URL
[Cite within: 1]
This work investigates the physical hemical evolution during artificial aging in water of four commercialized sunscreens containing TiO 2 -based nanocomposites. Sunscreens were analyzed in terms of mineralogy and TiO 2 concentration. The residues formed after aging were characterized in size, shape, chemistry and surface properties. The results showed that a significant fraction of nano-TiO 2 residues was released from all sunscreens, despite their heterogeneous behaviors. A stable dispersion of submicronic aggregates of nanoparticles was generated, representing up to 38 w/w% of the amount of sunscreen, and containing up to 30% of the total nano-TiO 2 initially present in the creams. The stability of the dispersion was tested as a function of salt concentration, revealing that in seawater conditions, a major part of these nano-TiO 2 residues will aggregate and sediment. These results were put in perspective with consumption and life cycle of sunscreens to estimate the amount of nano-TiO 2 potentially released into AQUATIC environment.
|
| [221] |
DOI:10.1016/j.envpol.2010.02.012
PMID:20346555
URL
[Cite within: 1]
Aging in water of a TiO(2)-based nanocomposite used in sunscreen cosmetics has been studied as a function of light and time. It consisted initially in a TiO(2) core, coated with Al(OH)(3) and polydimethylsiloxane (PDMS) layers. Size measurement, coating alteration, and surface charge were followed by laser diffraction, TEM/EDS, ICP-AES and electrophoretic mobility measurement. The nanocomposite rapidly underwent progressive dispersion in the aqueous phase, enabled by the dissolution of the PDMS layer. A stable suspension of colloidal byproducts from 50 to 700nm in size was formed. Their positively charged Al(OH)(3) surface was evidenced with an isoelectric point around 7-8, controlling the dispersion stability. The critical coagulation concentrations measured with NaCl and CaCl(2) was 2 10(-2) and 8 10(-3)M respectively. The presence of natural organic matter affected the colloidal stability according to the NOM/byproduct ratio. A 2 wt% ratio favored bridging flocculation, whereas a 20 wt% ratio induced sterical stabilization.
|
| [222] |
|