伴随着经济的发展,环境问题日趋严重。目前,我国水资源环境面临着十分严峻的问题,水污染现象严重,越来越多的污染物质排放到环境中,其中重金属和有机物污染问题尤其突出。水处理一直是环境保护中的一个难题,在解决目前人类所面临的各种环境问题的过程中,各类功能材料起着不可或缺的作用。新型环境功能纳米材料不仅在环境污染净化和环境修复方面起着重要的作用,而且在解决能源危机等方面也发挥着巨大的作用。纳米材料,是三维空间中至少有一维处于纳米尺度范围或者由该尺度范围的物质为基本结构单元所构成的材料的总称。按维数,纳米材料可以分为纳米薄膜、纳米线、纳米管、纳米粒子。纳米材料由于其尺寸很小,结构特殊,因此具有许多新的物理化学特性,如小尺寸效应、大的比表面、极高的反应活性等。这些特性使纳米科学已经成为当今世界上三大支柱科学(生命科学、信息科学、纳米科学)之一。
伴随着经济的发展,环境问题日趋严重。目前,我国水资源环境面临着十分严峻的问题,水污染现象严重,越来越多的污染物质排放到环境中,其中重金属和有机物污染问题尤其突出。水处理一直是环境保护中的一个难题,在解决目前人类所面临的各种环境问题的过程中,各类功能材料起着不可或缺的作用。新型环境功能纳米材料不仅在环境污染净化和环境修复方面起着重要的作用,而且在解决能源危机等方面也发挥着巨大的作用。纳米材料,是三维空间中至少有一维处于纳米尺度范围或者由该尺度范围的物质为基本结构单元所构成的材料的总称。按维数,纳米材料可以分为纳米薄膜、纳米线、纳米管、纳米粒子。纳米材料由于其尺寸很小,结构特殊,因此具有许多新的物理化学特性,如小尺寸效应、大的比表面、极高的反应活性等。这些特性使纳米科学已经成为当今世界上三大支柱科学(生命科学、信息科学、纳米科学)之一。
湖南大学环境科学与工程学院和环境生物与控制教育部重点实验室(湖南大学)长期从事纳米技术及其在环境修复领域的应用探索研究,研发了一系列新型纳米材料,通过对微观结构和纳米尺度效应等基础科学问题的研究,开发了一系列清洁、高效、环境友好型的环境功能材料,尽可能为环境修复提供有效的技术解决方案。研究领域涉及环境生物技术污染控制、环境污染控制电化学、纳米材料及水质净化与湖泊湿地污染修复、纳米材料环境效应研究等方向。由于在纳米材料的环境应用领域开展了大量研究工作,取得了一系列研究成果,因而于2012年撰写了题为 “Use of iron oxide nanomaterials in wastewater treatment: A review”的综述论文[1],发表在Science of the Total Environment期刊上。论文概述了氧化铁纳米材料的发展,介绍了氧化铁纳米材料的合成制备方法、在环境修复领域的应用性能以及可能存在的环境效应和环境风险,充分展望了氧化铁纳米材料在环境修复应用领域面临的问题以及可采取的各种策略。
氧化铁是指铁的氧化物以及羟基氧化物等氧化铁系列纳米材料,按价态、晶型和结构的不同可分为α-Fe2O3、β-Fe2O3、γ-Fe2O3、Fe3O4、FeO、和α-、β-、γ-FeOOH等。纳米级的氧化铁材料往往具有比氧化铁颗粒更好的物理和化学性质呈现出更广阔的应用潜力,故而受到了越来越多的关注[1]。在氧化铁材料中,γ-Fe2O3和Fe3O4具有磁性,是磁性纳米材料中非常重要的一种功能材料,其应用涉及到电子、信息、环境、交通、生物及医学等领域。当氧化铁颗粒尺寸减小到纳米级(1~100 nm)时,其表面原子数、比表面积和表面能等均随粒径的减小而显著增加,从而表现出表面效应、小尺寸效应、量子尺寸效应以及宏观量子隧道效应,具有良好的电磁、力学、光学等性能。
氧化铁纳米材料的性能取决于材料的粒径大小及其分布、化学组成、界面或表面的存在以及组分间的相互作用,而其结构与材料的组成和制备方法密切相关。氧化铁纳米材料的合成方法主要有物理法、化学法以及生物合成方法,国内外对氧化铁纳米材料的合成制备的研究多集中于通过控制合成过程以调控粒径大小及其分布、化学组成、界面特征等性能。以超顺磁性氧化铁纳米材料为例,最重要的方法为化学合成法,常见的化学合成法包括化学沉淀法、水热法、微乳液法、高温分解法以及超声分解法等。在合成过程中,通过不同方法以及合成过程各因素调节可以制备得到一系列具有不同粒径大小、磁性以及表面性能的超顺磁性氧化铁纳米材料(γ-Fe2O3和Fe3O4)。
氧化铁纳米材料由于尺寸小、比表面积大、表面可修饰强等特点,使其具有良好的吸附性能,在环境修复领域表现出极好的应用前景,也正受到愈来愈多的关注。目前,国内外关于氧化铁纳米材料在废水修复领域的应用研究主要集中在两个方向上:一是作为新型吸附剂应用于废水中有毒污染物的吸附去除,二是作为光催化材料催化降解废水中的有机污染物。
氧化铁纳米材料,尤其是磁性氧化铁纳米材料作为纳米吸附剂应用于废水中重金属和有机物的吸附去除得到了广泛研究。不同于传统吸附剂,磁性氧化铁纳米材料由于具有低扩散阻力,大比表面积,高吸附性能以及分离快速,回收再利用率高等优点,克服了传统吸附剂回收困难,二次污染等问题,被公认为是水处理中最理想的新型吸附材料之一。但是由于磁性氧化铁纳米粒子比表面积大,易因磁性吸引和范德华力作用产生聚沉和团聚现象,因此如何降低纳米粒子的表面能以提高磁性纳米粒子的分散性和稳定性成为了研究热点。常用的改变纳米材料表面性能的方法为表面修饰法,即通过物理化学方法在粒子表面引入新的官能团,使得粒子的空间位阻和静电斥力增大,改变其表面性能。目前常用方法有有机物修饰(在纳米粒子表面引入高密度的有机保护分子)和无机小分子功能化改性(磁性纳米材料与无机材料制备成磁性纳米复合材料)。氧化铁纳米材料常见的表面修饰方法为通过硅烷化或酯化反应共价修饰引入新的官能团(如—NH2、—OH、—COOH、—SH等)或在其表面包裹其他无机小分子材料(如SiO2)形成壳核结构,不仅可以改善粒子的稳定性和分散性,还可与污染物通过配位作用发生特异性结合使其具有一定的化学选择性,从而增加纳米粒子的吸附性能及吸附选择性,高效去除或回收水中的金属离子或难降解的有毒污染物。
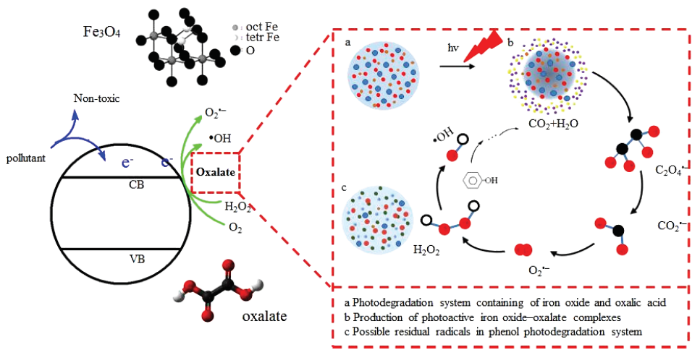
光催化氧化技术是近年来应用于环境污染处理的新兴技术,光催化过程是通过化学氧化过程将有机物完全降解成水和二氧化碳以及其他无机盐,是对污染物的深度矿化,具有处理速度快,简单易行、处理彻底以及经济适用等优势。氧化铁材料中,α-Fe2O3是n型半导体材料,带隙宽度较窄(2.2 eV),光响应波长较长,在可见光区域具有很强的光吸收能力,在可见光条件下可降解多种有机物(如苯酚。此外,铁作为氧化还原反应重要的变价元素,铁离子及其固态(氢)氧化物能参与均相、多相等催化作用,在光催化领域应用广泛。如Fe3O4纳米材料与小分子有机酸共存时可建立类光Fenton体系,该体系可以在普通日光灯照射、不加H2O2的情况下产生羟基自由基(•OH),•OH具有较高的氧化还原电位,能无选择地将水中难降解的污染物氧化为H2O、CO2和N2等无机小分子。图1为Fe3O4纳米颗粒与草酸共存体系下的类Fenton光催化氧化降解苯酚的过程[2]。
此外,由于氧化铁纳米材料具有较好的生物相容性,将其作为固定化载体应用于微生物的固定化技术也得到了广泛关注。2012年我们制备出了新型复合型磁性生物吸附剂,在白腐真菌菌球内部包埋可吸附并富集污染物的Fe3O4纳米粒子应用于废水中Pb(II)的吸附,Fe3O4纳米粒子固定化使生物吸附剂的机械强度和稳定性大大增强,有效增强了复合型磁性生物吸附剂的内部传输吸附机理,同时还可降低污染物对微生物的毒性[3,4],通过采用菌球包埋Fe3O4纳米粒子和海藻酸钙的结构形式,制备出了一种集物理、化学和生物吸附作用于一体的复合型吸附剂。同时,基于Fe3O4纳米粒子的生物相容性,我们还在黄孢原毛平革菌生长过程中加入Fe3O4纳米粒子,使其包裹于黄孢原毛平革菌菌球表面,菌球可正常生长,同时Fe3O4纳米粒子与黄孢原毛平革菌细胞外草酸作用,形成了生物降解-光催化复合体系应用于苯酚的可见光催化降解,具有处理效率高,无二次污染,投资少、易操作等优势[5]。
由于纳米技术和纳米材料所带来的可观的经济收益和技术进步,国内外的研究和相关投资都极为可观。然而这种新材料和新技术可能带来的生物安全性方面的影响以及相关的研究也逐渐被认识和重视。纳米技术安全性研究最早开始于1997年,随着纳米技术的迅猛发展,纳米技术安全性及与生物环境相互作用问题也受到越来越多的关注。纳米材料生物安全性是近年来的研究热点,纳米材料的广泛应用使得越来越多的人工合成纳米材料进入到环境中,在大气层、水圈和生物圈中传输转化。纳米材料进入环境后,可以不同的状态存在于环境中,并在各环境介质间迁移传输,一方面纳米材料的存在可改变环境中污染物的存在形态,改变污染物在环境中的迁移传输行为;另一方面,纳米材料本身可能存在的毒性效应可能对人体和生态环境造成一定的影响[6]。纳米材料可通过呼吸吸入、皮肤接触等进入人体,透过生物膜上进入细胞内在细胞器中富集,如人体摄入的纳米材料可进入线粒体、内质网、溶酶体和细胞核等细胞器内,并且和生物大分子发生结合或催化化学反应,改变生物膜以及其他生物大分子的结构,导致生物体内酶或激素的活性变化,从而产生毒性效应。相对于其他纳米材料,磁性氧化铁纳米粒子是由生物可降解铁合成得到的,具有较好的生物相容性。从目前已有的研究报道来看,氧化铁纳米材料的毒性相对较小,被人体摄入后可通过正常的生化代谢途径进入铁循环过程,但是仍不能忽视氧化铁纳米材料的安全性。因此,后续研究中,必须寻找纳米技术可能存在的潜在危害的解决办法,研制出生物相容性好,毒性较低的环境功能氧化铁纳米材料,满足氧化铁纳米材料在环境污染修复领域应用技术发展的同时尽量规避其环境风险和健康风险,加强纳米生物效应与安全性战略研究,为纳米技术的发展提供新的思路。
The authors have declared that no competing interests exist.
| [1] |
DOI:10.1016/j.scitotenv.2012.02.023
PMID:22391097
URL
[Cite within: 2]
Nowadays there is a continuously increasing worldwide concern for the development of wastewater treatment technologies. The utilization of iron oxide nanomaterials has received much attention due to their unique properties, such as extremely small size, high surface-area-to-volume ratio, surface modifiability, excellent magnetic properties and great biocompatibility. A range of environmental clean-up technologies have been proposed in wastewater treatment which applied iron oxide nanomaterials as nanosorbents and photocatalysts. Moreover, iron oxide based immobilization technology for enhanced removal efficiency tends to be an innovative research point. This review outlined the latest applications of iron oxide nanomaterials in wastewater treatment, and gaps which limited their large-scale field applications. The outlook for potential applications and further challenges, as well as the likely fate of nanomaterials discharged to the environment were discussed.
|
| [2] |
DOI:10.1039/c4ra05996d
URL
[Cite within: 1]
A novel approach for the removal of phenol by an advanced oxidation process using Fe3O4nanoparticles (NPs) and oxalate was proposed and investigated, and the influences of oxalate, Fe3O4NPs and H2O2dosage on the photodegradation of phenol were reported. No obvious difference is found between ultraviolet light and visible light exposure, confirmed potential photoactinic roles of Fe3O4NPs in the presence of oxalate under visible light. Furthermore, relatively high dependence of oxalate depletion was observed due to the initiation of the formation of the Fe(iii)-carboxylate complexes for photodegradationviaa photo-Fenton-like system. Our results also demonstrated that the photodegradation of phenol occurred by a radical mechanism accompanied with the formation of O261and OH radicals, which was further accelerated by the exogenous addition of H2O2. All reactions followed the pseudo-first-order reaction kinetics. The half-life (t1/2) of Fe3O4–oxalate and Fe3O4–oxalate–H2O2in the system showed higher efficiencies of photo-Fenton-like degradation routes for phenol. The photo-Fenton-like systems showed a relatively high catalytic ability (>99.9%) in the removal of phenol at low phenol concentrations below 50 mg L611, indicating its potential application in the treatment of low concentration wastewater. The results have demonstrated the feasibility of Fe3O4NPs as potential heterogeneous photo-Fenton photocatalysts for organic contaminants decontamination in industrial wastewater.
|
| [3] |
DOI:10.1016/j.colsurfa.2012.10.061
URL
[Cite within: 1]
A novel biosorbent was successfully prepared by the immobilization of Phanerochaete chrysosporium with iron oxide magnetic nanoparticles (MNPs) and Ca-alginate, which was confirmed by ESEM, EDS, FTIR and XRD characterization. Optimum biosorption conditions were determined as a function of pH, contact time and initial concentration of Pb(II). The maximum biosorption efficiency of Pb(II) was obtained at pH 5.0 and 3502°C, at the value of 96.03%. The uptake of metal was very fast initially, and achieved equilibrium after 802h. The maximum biosorption capacity reached up to 185.2502mg02g 611 dry biosorbent at a 50002mg02L 611 Pb(II)-containing sample. It was obvious that the prepared MNPs-Ca-alginate immobilized P. chrysosporium was capable of removing Pb(II) ions from solution efficiently, in terms of its performance and cost. According to Pearson correlation analysis, biosorption efficiency was mostly controlled by contact time and pH, but initial Pb(II) concentration also have a great effect on biosorption efficiency. While the temperature and biosorbent dosage affected at a lower extent. As a result, this work could provide a potential and unique technique for heavy metals removal by enhanced removal capacity and application stability.
|
| [4] |
DOI:10.1016/j.cej.2012.07.048
URL
[Cite within: 1]
A novel adsorbent, iron oxide magnetic nanoparticles and Ca-alginate immobilized Phanerochaete chrysosporium , was prepared for removal of Pb(II) ions. Confirmation of the experimental data indicated the pseudo-second-order reaction of the prepared adsorbents. Langmuir isotherm was found to be applicable in terms of relatively high correlation coefficients, which indicated that the adsorption between Pb(II) and the adsorbent was favorable. The thermodynamic analysis showed that the adsorption of Pb(II) was spontaneous and endothermic under the experimental conditions. The adsorption–desorption studies indicated that the prepared adsorbent kept its adsorption efficiencies constant over 5 cycles, maintained about 90%. All the results illustrated that MNPs–Ca-alginate immobilized P. Chrysosporium was a very attractive adsorbent for efficient Pb(II) removal from contaminated aqueous solution.
|
| [5] |
DOI:10.1016/j.ibiod.2014.11.001
URL
[Cite within: 1]
A novel composite system, containing FeOnanoparticles andtogether with its secretion oxalate, was developed for phenol degradation. FeOplayed an important role in the composite system as they could efficiently enhance phenol degradation when coexisted with oxalate under light. The maximal phenol degradation efficiency (93.41%) under solar light was detected at 0.5 g LFeO, while it reached a peak (40.36%) at 0.7 g LFeOunder dark. Additionally, oxalate was found to be strongly dependent on FeOnanoparticles concentration, ranging from 26.27 to 32.78 mM. And adequate oxalate could enhance phenol degradation due to the participation of oxalate in the photocatalytic process. Furthermore, phenol degradation efficiency increased with the increase of phenol concentration. Besides, the contribution of manganese peroxidase (MnP) to phenol degradation was greater than that of lignin peroxidase (LiP). Cluster analysis indicated that MnP and oxalate could be the main factors that influenced phenol degradation. Importantly, both the biodegradation and photocatalysis played part in the degradation process. These findings proposed a new method of treatment of phenol wastewater and could provide useful references for more efficient treatment of phenol wastewater.
|
| [6] |
DOI:10.1016/j.cis.2013.03.009
PMID:23642336
URL
[Cite within: 1]
Graphene, as an ideal two-dimensional material and single-atom layer of graphite, has attracted exploding interests in multidisciplinary research because of its unique structure and exceptional physicochemical properties. Especially, graphene-based materials offer a wide range of potentialities for environmental remediation and energy applications. This review shows an extensive overview of the main principles and the recent synthetic technologies about designing and fabricating various innovative graphene-based materials. Furthermore, an extensive list of graphene-based sorbents and catalysts from vast literature has been compiled. The adsorptive and catalytic properties of graphene-based materials for the removal of various pollutants and hydrogen storage/production as available in the literature are presented. Tremendous adsorption capacity, excellent catalytic performance and abundant availability are the significant factors making these materials suitable alternatives for environmental pollutant control and energy-related system, especially in terms of the removal of pollutants in water, gas cleanup and purification, and hydrogen generation and storage. Meanwhile, a brief discussion is also included on the influence of graphene materials on the environment, and its toxicological effects. Lastly, some unsolved subjects together with major challenges in this germinating area of research are highlighted and discussed. Conclusively, the expanding of graphene-based materials in the field of adsorption and catalysis science represents a viable and powerful tool, resulting in the superior improvement of environmental pollution control and energy development.
|